Neural Control of
Three-Dimensional Gaze Shifts
J. Douglas Crawford1
and Eliana M. Klier2
1.
York
Centre for Vision Research, Canadian Action and Perception Network,
Neuroscience Graduate Diploma Program, Depts. of Psychology, Biology and
Kinesiology & Health Sciences, York University, Toronto, Ontario M3J 1P3
2.
Dept.
of Anatomy and Neurobiology, Washington University School of Medicine, St.
Louis, Missouri 63108
Mailing
Address:
Dr.
Doug Crawford (jdc@yorku.ca)
York
Centre for Vision Research,
Room
1012B, Computer Science and Engineering Bldg.,
York
University,
4700
Keele Street, Toronto, Ontario, Canada, M3J 1P3
Phone:
(416) 736-2100 x88621
Fax:
(416) 736-5857
KEYWORDS: eye, head, torsion,
brainstem, cortex
ACKNOWLEDGMENTS: Supported by the Canadian
Institutes of Health Research and the Canada Research Chair Program
Abstract
In
laboratory conditions, with the head restrained and held upright, eye-in-head
orientation vectors are constrained to a tilted two-dimensional (2-D) range
called Listing’s plane. However, in most real-world conditions gaze control
utilizes a 3-D range. For example, when the head is allowed to move naturally,
the accompanying saccades and VOR movements include coordinated torsional
components; out of, and then back into Listing’s plane. The head itself rotates
more like a set of Fick Gimbals, resulting in a non-planar range of orientation
vectors. To control this complex behavior, the brainstem reticular formation
appears to have struck upon an elegant solution: it encodes the 3-D components
of posture and movement in coordinates that align with the Listing and Fick
behavioral constraints, such that its control signals collapse to 2-D (zero
torsion) when these constraints are upheld, but it retains the capacity for
torsional control whenever required. In contrast, the superior colliculus and
cortex appear to only encode 2-D gaze direction. Surprisingly, after many years
of research on this topic, we still know very little - other than a few clues -
about the neural mechanisms that transform high-level 2-D gaze direction
commands into the 3-D control signals for eye and head orientation.
Listing’s and
Donders’ laws
Most oculomotor studies are
primarily concerned with the control of 2-D gaze direction, i.e., how the brain
points the visual axis towards objects of interest. However there are two
important areas where one needs to consider the 3-D orientation of the eye. The
first involves any kind of visual stimulus that activates the retina beyond the
fovea, because here the spatial pattern of retinal stimulation depends both on
the configuration of the stimulus in space and the torsional orientation of the eyes around the visual axis. The
second (which will be the focus of this review) relates to gaze control: the eye is equipped with the
musculature to rotate in 3-D. As we shall see, the eyes can and do rotate about
nearly any combination of components about the vertical (left / right),
horizontal (up/down) and torsional (clockwise / counterclockwise) axes (Note
that directions are defined here from the subject’s perspective). As we shall
see, torsion is not allowed to vary randomly: the brain usually sets a certain
amount of torsion for a given 2-D gaze direction, and sometimes it actively generates
a muscular contraction to rapidly change the direction and amount of ocular
torsion.
In the 19th century
Donders proposed that for any one gaze direction, the eye assumes a unique
orientation (one torsional value), no matter how it got there (Donders 1848).
The precise value of this torsion was then described in Listing’s law. The
origins of Listing’s law are somewhat obscure. Listing was a German
mathematician who somehow intuited that the eye assumes only those orientations
that can be reached from some central reference position by rotations about
axes within a single plane. For one special reference position, the line of
gaze is orthogonal to the associate plane of axes; this is called primary
position, and the associated plane is called Listing’s plane. The best
coordinate system to describe Listing’s law (Listing’s coordinates) can be
defined by expressing eye orientations in terms of vectors aligned with the
axes of rotation from primary position, scaling these vectors to the angle of
rotation, and defining torsion as rotation about the head-fixed axis aligned
with the primary gaze direction. Once this coordinate system is defined,
Listing’s law is simple: it just says that torsion equals zero (Westheimer
1957). Examples of such data, in Listing’s coordinates, are provided in the
chapter by Angelaki and are also shown here in Figs. 3C and 6B.
The description of Listing’s
law in terms of axes of rotation is more complicated. Intuitively, one would
think that the eye would rotate about an axis in Listing’s plane, but this is
not what happens. In fact, if this is done, it causes a violation of Listing’s
law (Crawford and Vilis 1991). As illustrated in the companion chapter by Angelaki,
in order to keep eye position in Listing’s plane, the axis of eye rotation must
tilt out of Listing’s plane in a position-dependent manner. This is a
requirement of the laws of rotational kinematics (Tweed and Vilis 1986). (Many
people find that this makes intuitive sense only after a couple of years of
intense study; otherwise it is best left for mathematicians.)
Listing’s law was first
described, and confirmed, by Helmholtz, with the clever use of visual
after-images (von Helmholtz 1867). Modern recording techniques are more direct,
and usually involve the placement of two search coils in the eye within a set
of orthogonal magnetic fields. So far these experiments have not revealed any
behavioral differences between the human and monkey, so we will site literature
from both species together. These experiments have shown that Listing’s law is
obeyed when the head is held upright and stationary during saccades and
fixations (Ferman et al. 1987a, 1987b; Tweed and Vilis 1990; Straumann et al.
1990; Crawford and Vilis 1991). In monkeys Listing’s plane generally tilts back
in the head whereas in humans its orientation seems to vary highly from subject
to subject. Listing’s law is also obeyed during smooth pursuit eye movements
(Haslwanter et al. 1991; Tweed et al. 1992) and gaze fixation during purely
translational head movements (Angelaki 2000; Angekaki et al. 2003). It is
obeyed in modified form during vergence movements (where the Listing’s planes
of the two eyes tilt outward; Mok et al. 1992; Van Rijn et al.
1993) and when
the head is stationary but not upright (resulting in either shifts or tilts;
Crawford and Vilis 1991; Haslwanter et al. 1992). However, when the head
rotates, vestibular and / or visual inputs can stabilize the retinal image of
distant targets by rotating the eye about the same axis, but opposite
direction, as the head, thus violating Listing’s law (Crawford and Vilis 1991;
Fetter et al. 1992; Misslisch and Hess 2000). The common theme of these rules is that
whenever there is a degrees of freedom problem (gaze direction is specified but
not torsion) Listing’s law or some variant is used, but when specific torsional
movements are required Listing’s law is violated (Crawford et al. 2003).
There has been a surprisingly
long-lived, and often obscure controversy about whether Listing’s law is
implemented mechanically or neurally (Tweed and Vilis 1987; Tweed and Vilis
1990; Crawford and Vilis 1991; Schnabolk and Raphan 1994; Crawford and Guitton
1997; Quaia and Optican 1998; Raphan 1998; Misslisch H,
Tweed D. 2001; Angelaki
2003; Angelaki and Hess 2004). Rather than review this entire controversy we
will simply state our own view, which is that in restrospect this argument was
largely based on the conflation of two different computational issues. In order
to produce Listing’s law, the oculomotor system must do two things: it must
specify the desired 3-D orientation of the eye, and it then chose the correct
axis of eye rotation for a given initial position. In the first kinematically
correct model of the 3-D saccade generator, these two computations were done
‘neurally’ within one ‘Listing’s law box’. However, as described by Angelaki
elsewhere in this volume, there is good evidence to suggest that the
position-dependent axis tilts required to maintain eye position in Listing’s
plane are implemented mechanically by the tissues surrounding the eye (Demer et
al. 1995; 2002; Ghasia and Angelaki 2005; Klier et al. 2006). This
simplifies some of the control issues associated with generating eye movements
that stay within Listing’s plane. However, these mechanical position-dependencies
cannot constrain the eye to Listing’s
plane (if they did, the system would not be able to violate Listing’s law,
which is often does).
More recent models of the 3-D
saccade generator have separated the neural process of selecting the desired
orientation of the eye (and then choosing the movement vector that will get it
there) from the mechanical process that determines the required axis tilts
(Crawford and Guitton 1997; Tweed 1997; Glasauer et al. 2001a, 2001b). These
models demonstrate that an eye ‘plant’ optimized for Listing’s law will still
only produce Listing’s law if it is given the right neural signals, and will
violate Listing’s law if given different signals (Smith and Crawford 1998).
Thus, Listing’s law is both neural and mechanical: it is the sequential product
of neuromechanical control system.
Perhaps a second factor that
has skewed our view of Listing’s law is that 90% of the studies done on 3-D
ocular kinematics are done with the head artificially restrained. When the head
is allowed to move naturally, a different picture emerges.
What happens to these rules when the head
is not restrained?
Listing’s law is only upheld
continuously when the head is restrained. When the head is allowed to move
naturally the gaze control system shows quite different properties (again, the
story is quite similar for both the human and the monkey, so we will refer to
both literatures equally). Despite the additional complexity of eye-head
coordination, the system still appears to follow certain ‘lawful’ kinematic
relationships (see the chapter by Corneil in this volume), and 3-D control is no
exception. If one understands the oculomotor rules described in the previous
section, then one can understand the rules for eye-head coordination 1) by
understanding how these rules interact, and 2) by understanding the analogous
rules that apply to head movement.
Rule 1: as long as the
subject holds the eye and head stationary in space, the eye in-head range (Fig.
1 C) is statistically indistinguishable from Listing’s plane (Straumann et al.
1991; Glenn and Vilis 1992; Radeau et al. 1994; Crawford et al. 1999). However,
during gaze shifts torsional control
becomes more complicated. It is well known that during large rapid gaze shifts,
typically a visually-guided saccade occurs while the head is just starting to
build up momentum in the same general direction. Once the eye reaches its
target, the vestibular-ocular reflex turns on, causing the eye to roll back in
the head so that gaze stays on target while the head completes its trajectory
(Guitton 1992; see the companion chapter by Corneil for a more detailed review). The conundrum here for 3-D control is that,
as stated above, saccades obey Listing’s law and the VOR does not. Left
unchecked, the VOR would generally drive the eye quite far out of Listing’s
plane. Apparently to avoid this, during head-free gaze shifts saccades take on
anticipatory torsional components that are opposite and approximately equal to
the oncoming torsional component of the VOR (Crawford and Vilis 1991; Tweed et
al. 1997; Crawford et al. 1999). This results in the eye ending up back in
Listing’s plane when the whole sequence is done (Fig. 1 D). Tweed and
colleagues exploited this property to induced the ‘world record’ for eye
torsion in healthy subjects, inducing people to generate saccades with
torsional compoents up to 17º.Thus, continuous adherence to Listing’s law is an
artifact of immobilizing the head.
In terms of the head movement itself, during gaze
shifts the head follows its own version of Donders’ law, albeit less precisely
than the eye and in a different form (Glenn and Vilis 1992; Radeau et al. 1994;
Crawford et al. 1999). Instead of following Listing’s law, the head acts as if
it rotates horizontally about a body-fixed vertical axis but vertically about a
head-fixed horizontal axis, like a set of Fick Gimbals (Fig. 2). Torsion in
this new coordinate system is again kept at a minimum in Fick coordinates but
when these data are plotted in Listing’s coordinates this results in a non-planar
range of orientation vectors that is consistently twisted at the oblique
corners (Fig. 1B).
Like eye movements, head torsion is not always held
at zero, even in Fick coordinates: during oblique gaze shifts the head take the
shortest path from one corner of the range to the other in position space
rather than curving along its Donders’ range (Crawford et al. 1999). Moreover,
the Fick range is modified by gravity (shifting and tilting for body roll and
pitch, respectively) in a fashion similar to Listing’s law (Misslisch et al.
1994), can be modified to Listing’s plane when the head alone is used to point
gaze or to a shortest path strategy (resulting in a break down of Donders’ law)
when head pointing is dissociated from gaze (Ceylan et al. 2000). Thus, much
like Donders’ law for the eye, Donders’ law for the head is associated with its
own intrinsic coordinate system, where zero torsion can be selected, or not,
depending on task requirements.
The
final range to consider is that of eye orientation in space, which one can
think of as a 3-D version of gaze. Since Listing’s plane is fixed in the head,
and since eye position contributes relatively little to head-free gaze
fixations over a wide range, the eye and head constraints interact to produce a
range of eye-in-space (gaze) orientations that also resemble the range produced
by Fick Gimbals (Fig. 1 A). Moreover, since the control of head torsion is much
sloppier than control of eye torsion (in the order of ±5º compared to ±º1), the
resulting torsional range of the eye-in-space is even sloppier (Glenn and Vilis
1992; Crawford et al. 1999).
Premotor control of 3-D eye
velocity and orientation
Figure 3A shows the classic
Robinsonian model for oculomotor control (Robinson 1981). In this highly
influential model, eye movements are encoded by a velocity signal, that is then
integrated to provide a position signal, and the two are then summed at the
level of motoneurons to rotate the eye against resistive viscous forces and
hold it against elastic forces in the surrounding tissues. It turns out that
this model does not translate well into 3-D if the movement signal encodes
angular velocity (i.e., degrees / second about the physical axis of rotation)
because 3-D orientation is not the derivative of 3-D velocity (Tweed and Vilis
1987). For example, during saccades angular velocity has torsional components
(for the position-dependent axis tilts described above) that would be
integrated to produce inappropriate torsional position signals. However, if the
movement signal encodes derivatives - small changes in eye orientation divided
by time (Crawford 1994; Crawford and Guitton 1997; Quaia and
Optican 1998) - and feeds this to motoneurons for a
plant that mechanically implements the torsional axis tilts (Demer et al. 1995;
2002), then this scheme works just fine for saccades. Angelaki and colleagues
have verified this scheme by correlating motoneuron firing rate against the
torsional components of eye velocity (Ghasia and Angelaki 2005), and analyzing the changes
in eye position produced by motoneuron stimulation (Klier et al. 2006). This
works nicely for saccades, but complicates the VOR, which does not receive eye
orientation derivatives from the semicircular canals and does not like
position-dependent axis tilts (these would destabilize vision). However, it is a fairly simple matter for
the VOR to undo these tilts with the right interaction between eye position and
velocity signals before integration (Smith and Crawford 1998).
So where then do the premotor
signals arise for 3-D saccades and eye position? At this time, human brain
imaging techniques have too many spatiotemporal limitations to address this
question, so nearly everything we know about this system comes from
physiological studies with awake, behaving animals. The horizontal velocity
components of saccades are encoded by burst neurons in the paramedian pontine
reticular formation (PPRF) (e.g., Luschei and Fuchs 1972) and the corresponding
neural integrator for horizontal eye position is located in the nucleus
prepositus hypoglossi (NPH) (e.g., Cannon and Robinson 1987). The corresponding
circuits for vertical and torsional saccades are located in the midbrain (Fig.
6C). The rostral interstitial nucleus of the medial longitudinal fasciculus
(riMLF) possess burst neurons whose activity correlates to the vertical /
torsional components of rapid eye movements (Buttner et al. 1977; King et al.
1979; Hepp et al. 1988; Crawford and Vilis 1992). The interstitial nucleus of
Cajal (INC) appears to be the neural integration for vertical and torsional
components (Fukushima 1987; Crawford et al. 1991; Helmchen et al. 1998). It has
the right anatomy: the riMLF projects to the INC, and both project to the
motoneurons for eye muscles that control vertical / torsional rotation.
Moreover, we know of this arrangement because 1) the INC has activity related
to vertical / torsional eye position, 2) pharmacological inactivation
obliterates the ability to hold vertical / torsional eye positions (Fig. 3C),
and 3) electrical stimulation of the INC produces vertical / torsional eye
movements that hold their final position, as if one had ‘charged up’ a neural
integrator (Fig. 3B).
The riMLF and INC appear to be
similarly organized into pools of neurons with specific directional control very
similar to those of the eye muscles and semicircular canals (see chapter?).
Units on both sides of midline can be divided into randomly intermingled
populations with upward or downward velocity or position tuning. However these
same units are also tuned for clockwise components on the left side of midline
and counterclockwise components on the right side (Crawford et al. 1991;
Crawford and Vilis 1992). Taken together with the horizontal populations in the
PPRF / NPH, this creates a set of neuron pools like those illustrated in Fig.
4. This configuration is fully 3-D but
easily collapses to 2-D: whenever the oculomotor system requires a torsional
component (as in the saccades that occur with head-free gaze shifts) it need
only create an imbalance between activity in the left and right riMLF (and thus
INC) so that clockwise and counterclockwise components do not balance to zero.
But as long as these two sides are balanced (as in saccades with the head
fixed) torsion will cancel and the residual horizontal and vertical components
of activation will determine saccade direction (Crawford et al. 1991; Crawford
and Vilis 1992).
This is all very well, but
there is one hitch that is all too easy to take for granted. It depends on the
non-trivial assumption that the neuron pools in Fig. 4 are organized in a
coordinate system that aligns with Listing’s plane. It has been shown many
times that the orientation of Listing’s plane in the head varies considerably
from one subject to the next. If, for example, the PPRF encoded rotations about
an earth-vertical axis with the head upright, in most subjects PPRF activation
would drive eye position out of Listing’s plane. However, there is evidence
that these coordinates do in fact align. First, inactivation of the riMLF leaves
axes of rotation for horizontal saccades (presumably generated by the PPRF)
that align with Listing’s plane, and unilateral co-activation of the up and
down neuron populations of the riMLF produce rotations about an axis orthogonal
to Listing’s plane (Crawford and Vilis 1992). Third, torsional drift during
unilateral INC is perfectly orthogonal to Listing’s plane (Fig. 3C) and settles
to a range of positions parallel to Listing’s plane (Crawford 1994). Each of these observations is only possible
with the coordinate system we want: Listing’s coordinates.
To sum up, a series of
observations simplify the control of 3-D saccades. 1) eye muscles encode
derivatives, not angular velocity. 2) torsional control is arranged
symmetrically across the brainstem. 3) the brainstem coordinates for saccades
align with Listing’s plane. With this, and only this arrangement, saccades in
Listing’s plane will result from the planar coding of 2-D movement vectors. But
this still requires a very delicate balance of neural activation - no accident
or trivial default - and torsional saccade components must be programmed very
precisely when the head moves.
Premotor control
of head orientation
As with the eye, there may be
mechanical advantages for using a Fick-like coordinate system for head control.
For gaze shifts to distant targets, one is mainly concerned with head
orientation, but the head does not rotate in-place like the eye. Instead it
rotates (and translates) much like an inverted pendulum, except that base is a
flexible multi-jointed column (the cervical spine). The lower cervical
vertebrae act somewhat like the vertical axis (for horizontal rotation),
whereas vertical rotation (about the horizontal axis) occurs mainly about the
higher cervical vertebrae (Vidal et al. 1986; Graf et al. 1995), as in a nested
set of Fick axes. Nevertheless, it is once again clear that these mechanical
factors do not constrain the head to a zero-torsion range. To convince oneself
of this, one need only voluntarily roll the head torsionally from side to side.
This means that, as in the oculomotor system, the neural control system for the
head must be optimally matched to the mechanical stages of the control system.
Little is known about the
neural control of 3-D head orientation (head movement studies are essentially
impossible with current brain imaging techniques). However, there a few clues
from animal models that, at least so far as gaze control is concerned, the
oculomotor and head motor systems share both circuitry and control principles.
First of all, electrical stimulation of higher-level gaze structures in the
cortex, superior colliculus, and cerebellum evokes gaze shifts that involve
movements of both the eyes and head (this topic will be taken up further in the
next section). Moreover, this circuitry is shared down to the level of the
brainstem. For example, in animals with unilateral stimulation of most PPRF
sites produces ramp-like ipsilateral rotations of both the eyes and head
(Gandhi et al. 2008). This implicates the PPRF in the control of both eye and
head motion.
Similar observations have been
made for the INC. The INC projects to spinal cord neurons involved in neck
control via the intersitiospinal tract (Fukushima 1987; Fukushima et al. 1980,
1994). Unilateral stimulation of the INC (Fig. 5B) produces vertical /
torsional head rotations following very similar directions and patterns similar
to those seen in the eye (clockwise for left INC stimulation, counterclockwise
for right INC stimulation, and final positions that are held until corrected)
(Klier et al. 2002; 2007). As with the eye, unilateral inactivation of the INC
produces a transient nystagmus-like pattern of torsional head drift (Fig. 5A)
with corrective ‘quick phases’ that eventually dissipate, leaving the head
tilted in a torticollis-like posture (Klier et al. 2002; Farshadmanesh et al.
2007). These observations have led us to suggest that the INC is the 3-D
integrator not only for the eye, but also for head posture. However, head
control is much more complex than eye control - with vastly greater inertia, an
inverted pendulum structure, over-redundant musculature, multiple joints, and a
nest of vestibular and proprioceptive reflex pathways (Perlmutter et al. 1999;
Fukushima et al. 1994; Vidal et al. 1995) - one might better think of this
‘head integrator’ as determining a set-point for reflex control pathways.
We don’t know enough about
these head premotor circuits to say if they are organized into the same neuron
pools as the their corresponding oculomotor pools (or to what degree these eye
and head pools share member units) but what we know so far is consistent with
this notion. Moreover, there is also evidence that the head controller utilizes
a coordinate system aligned with the head’s Donders constraint. Following INC inactivation,
horizontal head positions continue to hold along the vertical axis of the Fick
coordinate (Klier et al. 2002; Farshadmanesh et al. 2007), and during
unilateral INC stimulation the head rotates about head-fixed horizontal axes –
like the vertical axis for head rotation in Fick coordinates (Fig. 5B) (Klier
et al. 2007). Thus, it appears likely that 1) both the eye and head are
controlled by neural populations organized into coordinates like those shown in
Fig. 4, and that 2) there is a continuous synergy between the neural,
mechanical, and behavioral coordinates for head control, which would have the
same advantages as described above for the eye.
Clinical
significance
One of the many things that
healthy people take for granted is that (other than some random scatter)
Donders’ laws of the eye and head are normally obeyed during gaze fixations.
However, this is not true in many clinical populations, for example those that
experience ocular tilt (a tonic torsional offset of the eyes; Westheimer et al.
1975; Halmagyi et al. 1991; Brandt 1992; Ohashi et al. 1998), torsional
nystagmus (torsional drift with intermittent corrective eye movements; Halmagyi
and Hoyt 1991; Straumann et al. 2000; Glasauer et al. 2001), and spasmodic
torticollis (Patterson and Little 1943; Medendorp et al. 1999; Agrawal et al.
2009). The latter (also known as cervical dystonia) is the most common type of
dystonia, and involves abnormal offset in head posture that very often have a
significant torsional component. Disorders of the motoneurons and eye muscles,
including strabismus, are also generally associated with abnormal torsional
positions (Sharpe et al. 2008). Each of these symptoms can be debilitating;
physically, functionally, emotionally, and socially.
Damage
or inappropriate activation of the reticular formation can explain some of
these symptoms, at least some of the time. This basic science review does not
have space for a comprehensive review of the clinical literature, but we can
highlight one particular example that ties directly in the previous physiology.
Acute unilateral damage to the INC produces an array of clinical symptoms
include vertical gaze-paretic nystamus (an inability to hold eccentric eye
positions), torsional nystagmus, and a combination of ocular tilt and
torticollis away from the damaged side). Each of these symptoms has been
associated with midbrain damage in the human.
It must be noted, when
comparing physiology to pathology, that most laboratory studies measure early,
acute, rapidly evolving affects that the clinician would rarely see. By the
time the patient reaches a specialist they have likely settled to a more
chronic state, perhaps even involving compensatory mechanisms. Moreover, nature
is unlikely to be as pin-point accurate in her neurological insults as
experimentalists are, so one needs to interpret patients in light of the
overall function of the damaged area. Finally, behaviors that resemble ocular
tilt and torticollis can also occur from unilateral INC stimulation (here on
the ipsilateral side to the tilt). This means that in pathological states,
structures such as these may be involved, but not the ultimate cause.
What
is coded at higher levels of the gaze control system?
In
the 1990s it was not known at what point in the gaze control system signals
became 3-D (i.e., included a specified torsional command). Clearly this is the
case in structures such as the INC and riMLF, but how far upstream does this
go? As mentioned above, the first kinematically correct model of the 3-D saccade
generator proposed that points on the superior colliculus (SC) encode specific
3-D saccade axes (Tweed and Vilis 1990), including the torsional axis
components required to keep eye position in Listing’s plane. However, Van
Opstal and colleagues showed, with a combination of unit recording and
microstimulation, that the SC does not encode 3-D axes (Van Opstal et al. 1991;
Hepp et al. 1993). Figure 6B replicates their result that stimulation of the SC
(in the head-fixed monkey) evokes saccades with zero torsional components (and
thus variable axes) independent of initial eye position. The latter results
seems less surprising two decades later, now that we know that the
position-dependent torsional axis tilts are still not implemented at the level
of motoneurons (Klier et al. 2006). However, we also know now that in natural
head-free conditions saccades are often accompanied by variable torsional
components. At what point in the system are these added on? Does the SC produce
a vector command with zero torsion, parallel to some other variable torsional
controller, or does the SC simply code a 2-D gaze target, which is then
converted somehow into a 3-D command downstream?
We tested this in a series of
experiments in the head-free monkey, in combination with electrical stimulation
of the SC, and several cortical gaze control structures, including the
supplementary eye fields (SEF) frontal eye fields (FEF) and lateral
intraparietal cortex (LIP). Stimulation of the SC, SEF, and FEF is known to
evoke gaze shifts that involve both eye and head movements. The simple logic
behind these experiments was that if the site encodes a specific amount of
torsion (whether zero or non-zero) stimulation should consistently evoke gaze
shifts with that same fixed torsional eye-in-head component. In general, this
would produce violations of Donders’ and Listing’s law (even if the saccades
had zero torsion). In contrast, if the site encodes 2-D gaze and 3-D control is
elaborated downstream, then stimulation should evoke gaze shifts with normally
coordinated torsional components in their saccades.
Figure 6 shows the typical result of
SC stimulation. The same site that produced zero-torsion saccades with the head
fixed produced saccades with torsional components with the head free, opposed
to the oncoming VOR components just as in normal head-free gaze shifts (Klier
et al. 2003). The final positions of the eye-in-head, eye-in-space, and
head-in-space obeyed Listing’s and Donders’ laws just as well as in normal
behavior (Fig. 6 A). Moreover, the eye-in-head showed the same pattern of
torsional coordination, with saccades showing anticipatory torsion
(interestingly, these stop when the head is fixed). We found the same results
in the SEF (Martinez-Trujillo et al. 2003) and FEF (Ascencio-Monteon et al.
2005). The exception so far has been LIP (Constantin et al. 2009): this
structure produces saccades with the correct torsion for an expected head-free
gaze shift, but then no head movement (and thus no VOR) occurs. This might
simply be because LIP is so far upstream from the premotor centres for eye and
head control that stimulation does not properly access the full motor circuitry
for a natural gaze shift. But in general, stimulation of high-level gaze
control structures suggests that they are only concerned with pointing gaze in
the right direction: 3-D control is elaborated at some point further downstream
(Fig. 6D).
The
2-D to 3-D transformation
The most interesting question
in 3-D gaze control remains to be solved: how are the higher-level 2-D signals
for gaze decomposed and elaborated into 3-D commands for eye and head rotation?
This gives rise to several sub-questions: how is zero torsion in Donders’
coordinates selected? How is this position range modified in behaviors that
follow a different variation of Donders’ law? How does the brain correct
torsional errors and select the right torsional saccade components to during
head-free gaze shifts?
To repeat, we are not looking
for the mechanism that causes saccade axes to tilt as a function of position:
as explained above, there is now good agreement that this is done by orbital
mechanics (Demer et al. 1995, 2002; Crawford and Guitton 1997; Tweed 1997;
Quaia and Optican 1998; Raphan 1998; Smith and Crawford 1998; Ghasia and
Angelaki 1995; Klier et al. 2006). Similarly, the neck may be mechanically
suited for the axes used in the Fick Strategy (Vidal et al. 1985; Graf et al.
1995).
What we are looking for is the
mechanism that actively chooses which Donders’ surface to use, when to modify
it, when to correct deviations from this range. One cannot dismiss the
theoretical possibility that eye muscle position-dependencies might be neurally
modified in ways that could modify Listing’s plane (Demer et al. 2000), but
this would require more, not less, neural complexity, and does not explain
active torsional control. We first need to understand the main mechanism that
sets torsional signals in the brainstem. It’s unlikely that this exists in a
single, separate ‘Listing’s law box’. For example, in neural networks trained
to perform these transformations the solution is distributed as torsional
modulations in units that are also involved in other functions (Smith and
Crawford 2005; Keith et al. 2007). Therefore, this may not be any easy process
to pin down. However, there are several clues.
One way to examine this is to start at both ends of the system and
see where 2-D meets 3-D. Searching from the highest level downward: if the
cortex and superior colliculus normally just encode 2-D gaze direction (Van Opstal
et al. 1991; Klier et al. 2003), then the 2-D to 3-D transformation for both
the eyes and head must occur downstream (closer to the muscles). Searching from the lowest level up: since
eye and head muscles, motoneurons, the neural integrator (INC and NHP), and
premotor burst neurons (riMLF and PPRF) can rotate the eye about any axis and
then hold it there (Hepp et al. 1988; Crawford and Vilis 1992), then the 2-D to
3-D transformation must occur at a functional level between the superior
colliculus and premotor burst neurons (Fig. 6C). Finally, since premotor burst
neuron (and neural integrator) coordinates align with Donders’ coordinates,
with clockwise and counterclockwise control symmetric across midline (Crawford
and Vilis 1992; Crawford 1994), the 2-D to 3-D transformation simplifies to
balancing torsion to zero during head-fixed saccades and smooth pursuit.
These factors suggest that
there may be a default mapping from 2-D superior colliculus outputs onto the
correct balance of burst neuron activity to encode zero torsion displacements
in Listing’s plane (and the Fick strategy for the head). However, this does not
explain how these strategies are modified, and how the system maintains these
ranges in the face of fairly common but brief violations. To do this, the
system requires a modifyable set-point (technically, a set-surface) with a comparator (Crawford and Guitton 1997; Ceylan et
al. 2000; Glasauer et al. 2001a, 2001b). In support of this, when the torsional
neural integrator is inactivated and the head is tilted, saccades keep aiming
the eye toward the torsionally shifted Listing’s plane even though the
integrator deficit will not allow it to hold there (Crawford et al. 2003). This
demonstrates that 1) the saccade generator actively maintains the desired set
point for torsion, and 2) this set point is modulated by vestibular inputs.
Furthermore, small errors in torsion (whether naturally or experimentally
induced) are usually corrected by forthcoming saccades (Van Opstal et al. 1995;
Lee et al. 2000) and these corrective components correlated to neural activity
in the nucleus reticularis tegmenti pontis (NRTP) (Van Opstal et al.
1995). Since this is a cerebellar input
nucleus, this implicates the cerebellum in the active control of torsion through
saccades. Consistent with this, patients with cerebellar damage show offsets
and widening of Listing’s planes (Straumann et al. 2000; Baier B, Dieterich M.
2009). Finally, another potential contributer is the central mesencephalic
reticular formation (cMRF) nucleus located just lateral to the INC, which has
functions related to saccades and eye-head coordination (Pathmanathan et al.
2006; Ugolini 2006), and has also been implicated in torticollis (Waitzman et
al. 2000), but its role in 3-D eye control is not known. We will not understand
the complete role of these structures until they are studied in 3-D / head free
preparations - where torsional control of the eye is most obvious and most
complex.
Finally, although this review
has focused on the control of 3-D gaze, as stated above, the 3-D orientations
of the eyes and head have extensive implications for higher level vision and
early aspects of gaze control. Because of the high variability of eye torsion
in space, and its effects on visual receptive fields (Keith et al. 2009), the
visual system must account both for systematic and variable torsion. For
example, the brain must monitor 3-D eye and head orientation to solve the
binocular correspondence problem (Blohm et al. 2008), and to convert eye-centered
visual information into useful commands for motor effectors (such as the eyes,
head, and limb) organized in head or body coordinates (Klier et al. 2001). Such
reference frame transformations can be done trivially - without comparisons
with position - in purely translational systems, but the eyes and head
primarily rotate. Thus, even when cortical mechanisms are primarily concerned
with aiming 2-D gaze direction (or depth), they cannot operate independently
from internal knowledge of 3-D eye & head orientation.
Conclusions
3-D gaze control is complex because it is not just the
control of torsion: it is the control of horizontal, vertical and torsional
components of rotation and all their interactions. Both theory and physiology
show that torsion cannot be neatly separated from the other components. This
3-D view forces us to give up comfortable intuitions grounded in translational
mathematics and leap into the odd, counter-intuitive world of rotational
kinematics. In this review, we have tried to illustrate that some aspects of
3-D control are mechanical and some are neural, but overall it must be
understood as a neuromechanical system. Moreover, 3-D gaze control is
inseparable from the topics of eye-head coordination, and visual-vestibular
integration. These factors combine to dictate that one cannot understand 3-D
gaze control without understanding the complete neurophysiology of gaze
control, and conversely, one cannot understand any component of this system
without understanding how it fits within the 3-D entirety.
References
Agrawal A, Cincu R, Joharapurkar SR,
Bhake A, Hiwale KM. 2009. Hemorrhage in brain stem cavernoma presenting with
torticollis. Pediatr Neurosurg 45: 49-52.
Angelaki DE.
2003. Three-dimensional ocular kinematics during eccentric rotations: evidence
for functional rather than mechanical constraints. J Neurophysiol 89: 2685-2696.
Angelaki DE,
Hess BJ. 2004. Control of eye orientation: where does the brain's role end and
the muscle's begin? Eur J Neurosci
19: 1-10.
Angelaki DE,
Zhou HH, Wei M. 2003. Foveal versus full-field visual stabilization strategies
for translational and rotational head movements. J Neurosci 23: 1104-1108.
Baier B, Dieterich M. 2009.
Ocular tilt reaction: a clinical sign of cerebellar infarctions?
Neurology 72: 572-573.
Blohm G,
Khan AZ, Ren L, Schreiber KM, Crawford JD. 2008. Depth estimation from
retinal disparity requires eye and head orientation
signals. J Vis 8: 1-23.
Buttner U, Buttner-Ennever JA, Henn V. 1977. Vertical eye unit related activity in the
rostral mesencephalic reticular formation of the
alert monkey. Brain Res 130: 239-252
Brandt T, Strupp M.
1992. Otoneurology.
Curr Opin Neurol Neurosurg. 5: 727-732.
Cannon SC,
Robinson DA. 1987. Loss of the neural integrator of the oculomotor system from
brain stem lesions in monkey. J
Neurophysiol 57: 1383-1409.
Ceylan M, Henriques DY, Tweed
DB, Crawford JD. 2000. Task-dependent constraints
in motor control: pinhole goggles make the head move like
an eye. J Neurosci
20: 2719-2730.
Constantin AG, Wang H, Monteon JA,
Martinez-Trujillo JC, Crawford JD. 2009. 3-D eye-head coordination in gaze
shifts evoked during stimulation of the Lateral Intraparietal cortex (LIP). Neuroscience in press.
Crawford JD. 1994. The
oculomotor neural integrator uses a behavior-related
coordinate system. J Neurosci 14: 6911-6923.
Crawford JD, Cadera W, Vilis
T. 1991 Generation of torsional and vertical eye
position signals by the interstitial nucleus of Cajal. Science
252: 1551-1553.
Crawford JD, Ceylan MZ, Klier EM, Guitton
D. 1999. Three-dimensional eye-head coordination during gaze saccades in the
primate. J Neurophysiol 81:
1760-1782.
Crawford JD,
Guitton D. 1997. Visual-motor transformations required for accurate and
kinematically correct saccades. J
Neurophysiol 78: 1447-1467.
Crawford JD, Tweed DB, Vilis
T. 2003. Static ocular counterroll is implemented
through the 3-D neural integrator. J
Neurophysiol 90: 2777-2784.
Crawford JD, Vilis T. 1991. Axes of eye rotation and Listing's law
during rotations of the head. J Neurophysiol
65: 407-423.
Crawford JD, Vilis T. 1992.
Symmetry of oculomotor burst neuron coordinates about
Listing's plane. J Neurophysiol 68: 432-448.
Demer JL. 2002.
The orbital pulley system: a revolution in concepts of orbital anatomy. Ann N Y Acad Sci 956: 17-32.
Demer JL, Clark
RA. 2005. Magnetic resonance imaging of human extraocular muscles during static
ocular counter-rolling. J Neurophysiol 94: 3292-3302.
Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. 1995. Evidence
for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci 36: 1125-1136.
Demer JL, Oh
SY, Poukens V. 2000. Evidence for active control of rectus extraocular muscle
pulleys. Invest Ophthalmol Vis Sci 41: 1280-1290.
Donders FC. 1848. Beitrag
zur lehre von den bewegungen des menschlichen auges.
[translation: The movements of the human eye] Holländ
Beitr Anat Physiol Wiss 1:
104-145.
Farshadmanesh F, Klier EM,
Chang P, Wang H, Crawford JD. 2007. Three-dimensional
eye-head coordination after injection of muscimol into
the interstitial nucleus
of Cajal (INC). J Neurophysiol 97: 2322-2338.
Ferman L, Collewijn H, Van den Berg AV.
1987. A direct test of Listing's law--I. Human
ocular torsion measured in static
tertiary positions. Vision Res 27:
929-938.
Ferman L, Collewijn H, Van den
Berg AV. 1987. A direct test of Listing's law--II. Human
ocular torsion measured under dynamic conditions. Vision
Res. 27: 939-951.
Fetter M, Tweed D, Misslisch H, Fischer D,
Koenig E. 1992. Multidimensional
descriptions of the optokinetic and
vestibuloocular reflexes. Ann N Y Acad
Sci 656: 841-842.
Fukushima K. 1987.
The interstitial nucleus of Cajal and its role in the control of
movements
of head and eyes. Prog Neurobiol 29: 107–192.
Fukushima K, Murakami
S, Matsushima J, Kato M. 1980. Vestibular responses and
branching
of interstitiospinal neurons. Exp Brain Res 40: 131–145.
Fukushima
K, Ohashi T, Fukushima J. 1994. Effects of chemical deactivation of the
interstitial
nucleus of Cajal on the vertical vestibulo-collic reflex induced by pitch
rotation in alert cats. Neurosci Res 20: 281–286.
Fukushima
K, Takahashi K, Kudo J, Kato M. 1985. Interstitial-vestibular interaction in
the
control of head posture. Exp Brain Res 57: 264–270.
Gandhi NJ, Barton EJ, Sparks
DL. 2008. Coordination of eye and head components of
movements evoked by stimulation of the paramedian pontine
reticular formation.
Exp Brain Res 189: 35-47.
Ghasia FF, Angelaki DE. 2005. Do motoneurons
encode the noncommutativity of ocular
rotations? Neuron 47: 281-293.
Glasauer S,
Dieterich M, Brandt T. 2001. Modeling
the role of the interstitial nucleus of
Cajal in otolithic control of
static eye position. Acta Otolaryngol Suppl 545: 105-107.
Glasauer S, Dieterich M,
Brandt T. 2001. Central positional nystagmus simulated by a
mathematical ocular motor model of otolith-dependent
modification of Listing's
plane. J Neurophysiol 86: 1546-1554.
Glenn B,
Vilis T. 1992. Violations
of Listing's law after large eye and head gaze shifts. J
Neurophysiol 68: 309-318.
Graf W, de Waele C, Vidal PP.
1995. Functional anatomy of the head-neck movement
system of quadrupedal and bipedal mammals. J Anat
186: 55-74.
Graf W, de Waele C, Vidal PP,
Wang DH, Evinger C. 1995. The orientation of the
cervical vertebral column in unrestrained awake animals.
II. Movement
strategies. Brain Behav Evol 45: 209-231.
Graf WM, Ugolini G. 2006. The
central mesencephalic reticular formation: its role in
space-time coordinated saccadic eye movements. J
Physiol 1; 570: 433-434.
Guitton D. 1992. Control of
eye-head coordination during orienting gaze shifts.
Trends Neurosci 15: 174-179.
Halmagyi GM, Curthoys IS, Brandt T,
Dieterich M. 1991. Ocular tilt reaction:
clinical sign of vestibular lesion. Acta Otolaryngol Suppl 481: 47-50.
Halmagyi GM, Hoyt WF. 1991. See-saw
nystagmus due to unilateral mesodiencephalic
lesion. J Clin Neuroophthalmol
11: 79-84.
Haslwanter T, Straumann D, Hepp K, Hess BJ, Henn V. 1991. Smooth
pursuit eye movements obey Listing's law in the monkey. Exp Brain Res 87: 470-472.
Haslwanter T, Straumann D, Hess BJ, Henn V. 1992. Static roll and
pitch in the monkey: shift and rotation of Listing's plane. Vision Res 32: 1341-1348.
Helmchen C, Rambold H, Fuhry
L, Büttner U. 1998. Deficits in vertical and torsional
eye movements after uni- and bilateral muscimol
inactivation of the interstitial
nucleus of Cajal of the alert monkey. Exp Brain Res
119: 436-452.
Helmholtz H. 1867. Handbuch der
Physiologischen Optik [Treatise of optical physiology] Treatise on
Physiological Optics 3(1). Hamburg: Voss;. (English Translation),
vol 3. Translated by JPC Southall. Rochester: Optical Society of America; 1925:
44-51.
Henn V,
Büttner-Ennever JA, Hepp K. 1982. The primate oculomotor system. I.
Motoneurons. A synthesis of
anatomical, physiological, and clinical data. Hum Neurobiol 1: 77-85.
Hepp K, Van Opstal AJ, Straumann D, Hess BJ, Henn V.
1993. Monkey superior
colliculus
represents rapid eye movements in a two-dimensional motor map. J Neurophysiol 69: 965-979.
Hepp K, Vilis T, Henn V. 1988.
On the generation of rapid eye movements in three
dimensions. Ann N Y Acad Sci 545: 140-153.
Keith GP, Desouza JF, Yan X,
Wang H, Crawford JD. 2009. A method for mapping
response fields and determining intrinsic reference
frames of single-unit
activity: applied to 3D head-unrestrained gaze shifts. J
Neurosci Methods
180: 171-184.
Keith GP, Smith MA, Crawford
JD. 2007. Functional organization within a neural
network trained to update
target representations across 3-D saccades. J Comput Neurosci 22:
191-209.
King WM, Fuchs AF. 1979.
Reticular control of vertical saccadic eye movements by
mesencephalic burst neurons. J Neurophysiol 42:
861-876.
King WM, Fuchs AF, Magnin M.
1981. Vertical eye movement-related responses of
neurons in midbrain near
intestinal nucleus of Cajal. J Neurophysiol 46: 549-562.
Klier EM,
Crawford JD. 1998. Human oculomotor system accounts for 3-D eye orientation in
the visual-motor transformation for saccades. J Neurophysiol 80: 2274-2294.
Klier EM, Meng
H, Angelaki DE. 2006. Three-dimensional kinematics at the level of the
oculomotor plant. J Neurosci 26: 2732-2737.
Klier EM,
Wang H, Constantin AG, Crawford JD. 2002. Midbrain control of
three-dimensional head orientation. Science. 295:
1314-1316.
Klier EM, Wang H, Crawford JD.
2003. Three-dimensional eye-head coordination is
implemented downstream from the superior colliculus. J
Neurophysiol
89: 2839-2853.
Klier EM,
Wang H, Crawford JD. 2007. Interstitial
nucleus of cajal encodes
three-dimensional head orientations in Fick-like coordinates.
J Neurophysiol
97: 604-617.
Kono R, Clark RA, Demer JL. 2002. Active
pulleys: magnetic resonance imaging of
rectus muscle paths in tertiary
gazes. Invest Ophthalmol Vis Sci 43:
2179-2188
Lee C, Zee DS, Straumann D.
2000. Saccades from torsional offset positions back to
listing's plane. J Neurophysiol 83: 3241-3253.
Luschei ES,
Fuchs AF. 1972. Activity
of brain stem neurons during eye movements of
alert monkeys. J Neurophysiol 35: 445-461.
Martinez-Trujillo JC,
Medendorp WP, Wang H, Crawford JD. 2004. Frames of reference
for eye-head gaze commands in
primate supplementary eye fields. Neuron 44: 1057-1066.
Martinez-Trujillo JC, Wang H,
Crawford JD. 2003. Electrical stimulation of the
supplementary eye fields in
the head-free macaque evokes kinematically normal
gaze shifts. J Neurophysiol 89: 2961-2974.
Medendorp WP, van Gisbergen JA, Horstink MW, Gielen CC.
1999. Donders'
law in
torticollis. J Neurophysiol 82: 2833-2838.
Minken AW, Van Gisbergen JA. 1994. A
three-dimensional analysis of vergence
movements at various levels of elevation.
Exp Brain Res 101: 331-345.
Misslisch H, Hess BJ. 2000. Three-dimensional vestibuloocular reflex
of the monkey: optimal retinal image stabilization versus listing's law. J Neurophysiol 83: 3264-3276.
Misslisch H, Tweed
D. 2001. Neural and mechanical factors in eye control. J Neurophysiol 86: 1877-1883.
Misslisch H,
Tweed D, Fetter M, Vilis T. 1994. The influence of gravity on Donders'
law for head movements. Vision Res 34: 3017-3025.
Mok D, Ro A, Cadera W, Crawford JD, Vilis T.
1992. Rotation of Listing's plane during
vergence. Vision Res 32: 2055-2064.
Monteon JA, Wang H,
Martinez-Trujillo JC, Crawford, JD. 2005. Gaze shifts evoked by electrical
stimulation of the frontal eye field in the head-free macaque. Soc Neurosci Abst
Ohashi T, Fukushima K, Chin S,
Harada T, Yoshida K, Akino M, Matsuda H. 1998.
Ocular tilt reaction with
vertical eye movement palsy caused by localized unilateral midbrain lesion. J
Neuroophthalmol 18: 40-42.
Pathmanathan
JS, Presnell R, Cromer JA, Cullen KE, Waitzman DM. 2006.
Spatial characteristics of
neurons in the central mesencephalic reticular formation (cMRF) of
head-unrestrained monkeys. Exp Brain Res 168: 455-470
Patterson RM, Little
SC. 1943. Spasmodic torticollis. Journal of Nervous and Mental
Disease
98: 571-599.
Perlmutter SI,
Iwamoto Y, Baker JF, Peterson BW. 1999. Spatial alignment of rotational
and
static tilt responses of vestibulospinal neurons in the cat. J Neurophysiol
82: 855–862
Peterson BW. 2004.
Current approaches and future directions to understanding control
of
head movement. Prog Brain Res 143: 369–381.
Porrill J, Warren PA, Dean P. 2000. A
simple control law generates Listing’s positions in a detailed model of the
extraocular muscle system. Vision Res
40: 3743-3758.
Quaia C,
Optican LM. 1998. Commutative saccadic generator is sufficient to control a 3-D
ocular plant with pulleys. J Neurophysiol
79: 3197-3215.
Radau P, Tweed D, Vilis T.
1994. Three-dimensional eye, head, and chest orientations
after large gaze shifts and
the underlying neural strategies. J Neurophysiol 72: 2840-2852.
Raphan T. 1998.
Modeling control of eye orientation in three dimensions. I. Role of muscle
pulleys in determining saccadic trajectory. J
Neurophysiol 79: 2653-2667.
Robinson DA.
1981. The use of control systems analysis in the neurophysiology of eye
movements. Annu Rev Neurosci 4: 463-503.
Scherberger H, Cabungcal JH, Hepp K, Suzuki Y, Straumann D, Henn V. 2001. Ocular
counterroll modulates the preferred direction of saccade-related pontine burst
neurons in the monkey. J Neurophysiol
86: 935-949.
Smith MA,
Crawford JD. 1998. Neural control of rotational kinematics within realistic
vestibuloocular coordinate systems. J
Neurophysiol 80: 2295-2315.
Smith MA, Crawford JD. 2005.
Distributed population mechanism for the 3-D
oculomotor reference frame transformation. J
Neurophysiol 93: 1742-1761.
Schnabolk C, Raphan T. 1994.
Modeling three-dimensional velocity-to-position
transformation in oculomotor control. J Neurophysiol
71: 623-638.
Sharpe
JA, Wong AM, Fouladvand M. 2008. Ocular motor nerve palsies: implications
for diagnosis and mechanisms of repair. Prog Brain Res
171: 59-66.
Straumann
D, Haslwanter Th, Hepp-Reymond MC, Hepp K. 1991. Listing's law for the
eye, head and arm movements and their synergistic control. Exp. Brain
Res. 86: 209-215.
Straumann D,
Zee DS, Solomon D. 2000. Three-dimensional
kinematics of ocular drift
in humans with cerebellar atrophy. J
Neurophysiol 83: 1125-1140.
Suzuki Y, Straumann D, Simpson JI, Hepp
K, Hess BJ, Henn V. 1999. Three-
dimensional extraocular motoneuron
innervation in the rhesus monkey. I: Muscle rotation axes and on-directions
during fixation. Exp Brain Res 126:
187-199.
Tweed D, Fetter
M, Andreadaki S, Koenig E, Dichgans J. 1992. Three-dimensional properties of
human pursuit eye movements. Vision Res
32: 1225-1238.
Tweed D,
Misslisch H, Fetter M. 1994. Testing models of the oculomotor
velocity-to-position transformation. J
Neurophysiol 72: 1425-1429.
Tweed D, Vilis
T. 1987. Implications of rotational kinematics for the oculomotor system in
three dimensions. J Neurophysiol 58:
832-849.
Tweed D, Vilis
T. 1990. Geometric relations of eye position and velocity vectors during
saccades. Vision Res 30: 111-127.
Van Opstal AJ, Hepp K, Hess BJ, Straumann D, Henn V. 1991. Two-
rather than three-dimensional representation of saccades in monkey superior
colliculus. Science 252: 1313-1315.
Van Opstal J,
Hepp K, Suzuki Y, Henn V. 1996. Role of monkey nucleus reticularis tegmenti
pontis in the stabilization of Listing's plane. J Neurosci 16: 7284-7296.
Van Rijn LJ,
Van den Berg AV. 1993. Binocular eye orientation during fixations: Listing's
law extended to include eye vergence. Vision
Res 33: 691-708.
Vidal PP, Graf W, Berthoz A.
1986. The orientation of the cervical vertebral column in
unrestrained awake animals. I. Resting position. Exp
Brain Res 61: 549-559.
Waitzman DM, Silakov VL,
DePalma-Bowles S, Ayers AS. 2000. Effects of reversible
inactivation of the primate mesencephalic reticular
formation. I. Hypermetric
goal-directed saccades. J
Neurophysiol 83: 2260-2284.
Westheimer G, Blair SM. 1975. The ocular tilt reaction--a brainstem
oculomotor
routine. Invest Ophthalmol 14: 833-839.
Westtheimer G. 1957. Kinematics of the eye. J Opt Soc Am 47: 967-974.
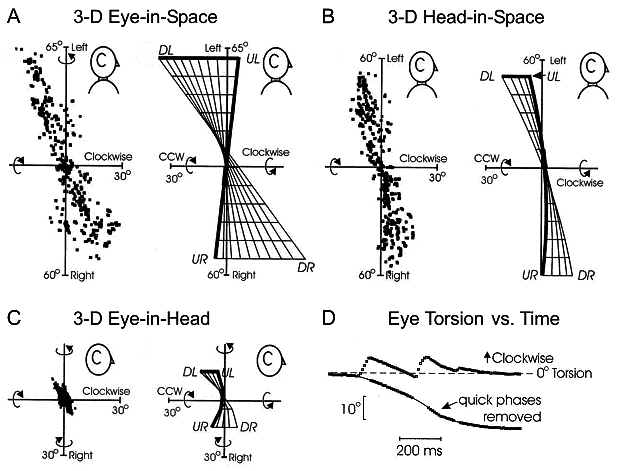
Fig. 1. Donders’ laws for eye, head, and
eye-in-space during head-free gaze fixations in the monkey (human data look the
same). In each case torsional orientation is restricted compared to vertical
and horizontal orientation. Panels A-C
(left sides) plot tips of 3-D eye position vectors (horizontal component as a
function of torsional component) in an orthogonal right-hand coordinate system.
The right sides show 2-D surfaces fit to these ranges. In the 3-D gaze
literature this is known as a ‘side view’, because it views axes of rotation
(relative to the zero vector reference position) are viewed from the side. DL, UL, UR, and DR represent Down-Left, Up-Left, Up-Right, and Down-Right
orientations. A: The range of eye
orientation in space consistently follows a twisted ‘Fick’ range. B: The range of head orientation in
space also follows a Fick range. C:
The range of eye orientation relative to the head shows variable twists that is
not significantly different from the (Listing’s) planar range seen in
head-fixed saccades. D: Torsional
eye-in-head position plotted during one multi-step gaze shift including a large
horizontal head movement (not shown). Without the anticipatory torsional
components in saccades (quick phases) the eye would be driven far from
Listing’s plane. Adapted from Crawford et al. 1999.
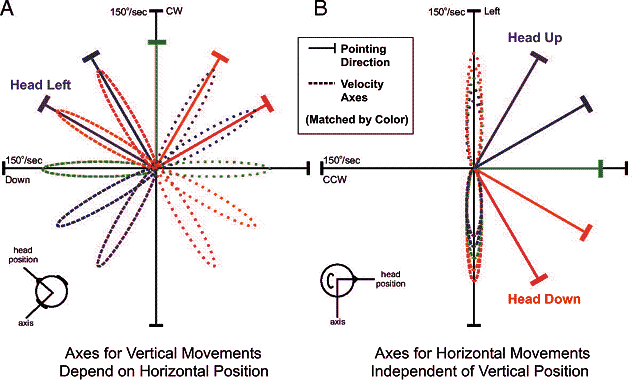
Fig. 2. Schematic axes of rotation, and their
dependence on orientation, in a Fick system, plotted in orthogonal Cartesian
coordinates. Angular velocity (broken lines) is a vector parallel to the axis
of rotation, scaled by the speed of rotation.
The angular velocity of the eye and head generally follows the loops,
starting at zero velocity, growing to maximum velocity, and then returning to
zero. In each panel, five head pointing directions are shown (solid lines),
each color coded to two velocity loops in opposite directions (dashed vs.
dotted lines). A: Velocity loops for
vertical rotations at five horizontal positions, viewed from above. B: Velocity loops for horizontal
rotations at five vertical positions, viewed from the side. Real head data
follow the same pattern, but are never as symmetric. Adapted from Klier et al.
2007.
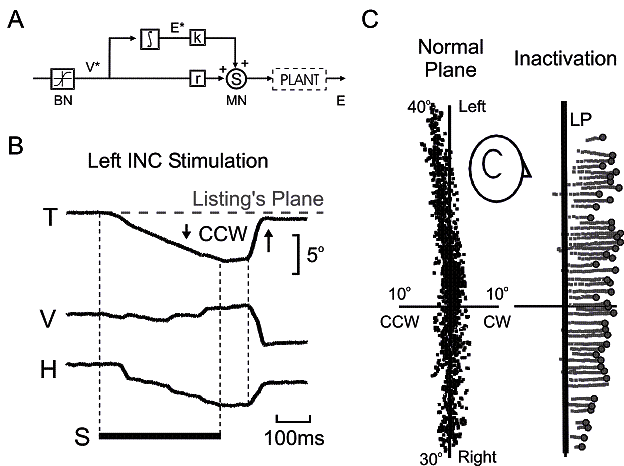
Fig. 3. Evidence of a 3-D neural integrator for
eye orientation, organized in Listing’s coordinates, in the interstitial
nucleus of Cajal (INC). A: David A.
Robinson’s seminal 1-D model of the saccade generator. Reticular formation
burst neurons (BN) have a saturating
estimate of desired eye velocity (V*) which is sent to the neural integrator
(∫), which converts this into a desired eye position signal (E*). V* and
E* are then scaled and summed at the motoneurons (MN) to provide the required signal to control the PLANT (eye and muscles). B: Torsional (T), vertical (V) and
horizontal (H) components of eye position plotted against time during INC
stimulation (S). The eye rotates primarily counterclockwise (CCW) or clockwise
(CW) out of Listing’s plane during left and right INC stimulation respectively.
Final eye position is held until the next saccade, which returns it to
Listing’s plane. Similar data published in Crawford et al. 1991. C: Left side: eye orientation vectors
during head-fixed fixations, plotted in Listing’s coordinates and viewed from
the side. Right panel: following injection of muscimol into the the left INC,
the eye drifts (gray traces) clockwise, orthogonal to Listing’s plane (LP)
until the start of the next saccade (○). Right INC injection produces the
opposite pattern. Similar data plotted in Crawford 1994.
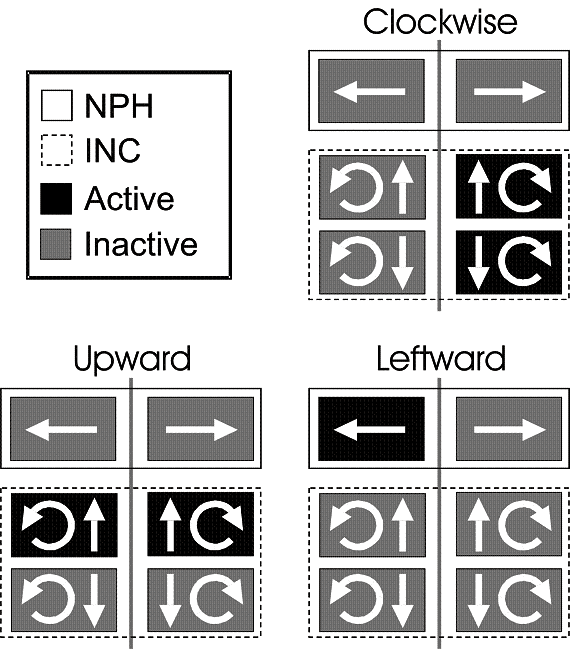
Fig. 4. Schema of populations of neurons for
eye and head orientation control in the nucleus preopositus hypoglossi (NPH)
and interstitial nucleus of Cajal (INC). Six neural populations are shown,
divided across the brainstem midline (vertical bar) with arrows indicating
their directional control in a fashion very similar to the semicircular canals
and eye muscles (where vertical and torsional components are combined). The filled color blocks show how these
populations would be activated during clockwise (upper right panel), upward
(lower left panel), and leftward (lower right panel) orientations. This schema
only works if these population coordinate align with the intrinsic coordinates
of behavior, i.e., Listing’s plane for the eye and Fick coordinates for the
head. A similar organization is seen in the burst neurons that provide the
velocity signal for the eye. Adapted from Crawford and Vilis 1992.
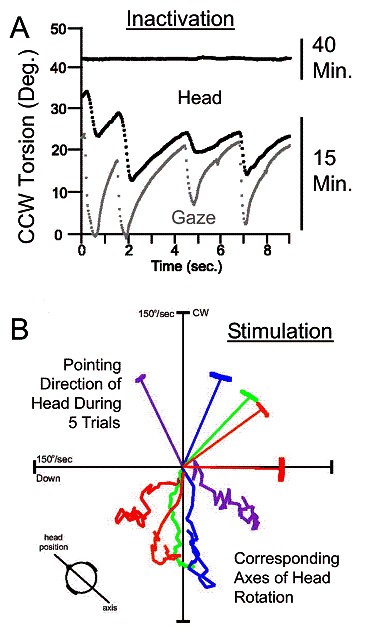
Fig. 5. Evidence of a neural integrator for
head orientation in the INC, organized in Fick coordinates. A: Head (dark line) and gaze /
eye-in-space (gray line) torsion plotted against time following muscimol
injection. Shortly after injection (15 minutes here) both drift away from the
regular upright position, while rapid movements attempt to correct this. Later
(40 minutes here) the head settles in a torsionally shifted position,i.e.,
corrective movements cease. Adapted from Klier et al. 2002. B: During unilateral INC stimulation with
the head free, the head rotates around vertical-torsional axes (clockwise for
left INC; counterclockwise for right INC) that stay fixed relative to
horizontal head orientation, as in a Fick gimbal. Conventions similar to Fig.
2A, but here real data are shown. Adapted from Klier et al. 2007.
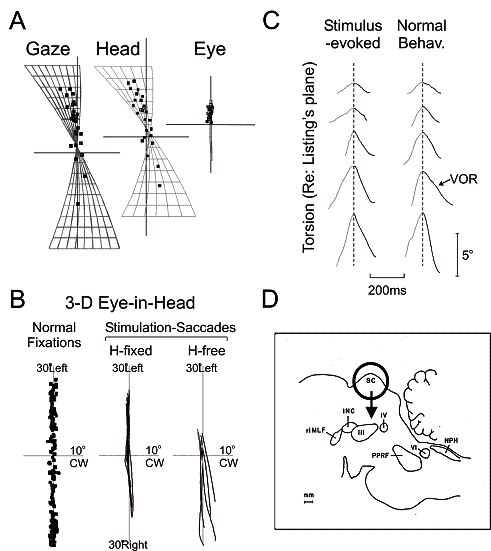
Fig. 6. Stimulation of the superior colliculus
(SC) produces gaze shifts with normal 3-D kinematics. A: At the end of stimulation-evoked movements, Gaze (eye-in space),
Head, and Eye orientation vectors fall within the normal Donders’ ranges
(compare to Fig. 1 A-C). B: When the
head is immobilized, stimulation-evoked saccades (center plot) stay within the
normal Listing’s plane range (left plot), viewed here from the side. When the
head is freed, stimulation of the same site produces saccades (right plot) that
flare out of Listing’s plane. Why? C:
Plotting torsion against time, one can see that both head-free stimulation
evoked saccades (left plots) and normal saccades (right plots) show the same
pattern of anticipatory torsion (gray traces): negating the torsion in the
following VOR (black traces). A-C adapted from Klier et al. 2003. D: Schematic saggital slice of monkey
brainstem. The previous data suggest that the SC (black circle) encodes desired
2-D gaze, and this is somehow elaborated into 3-D commands at the level of the
rostral intersititial nucleus of medial longitudinal fasciculus (riMLF),
interstitial nucleus of Cajal (INC), paramedian pontine reticular formation
(PPRF) and nucleus prepositus hypoglossi (NPH). III, IV, VI: 3rd, 4th, 6th
cranial nuclei contain motoneurons for eye muscles. Adapted from Henn et al.
1982.