ATKINSON FACULTY OF LIBERAL AND PROFESSIONAL STUDIES
SCHOOL OF ANALYTIC STUDIES & INFORMATION TECHNOLOGY
S C I E N C E A N D T E C H N O L O G Y S T U D I E S
NATS 1800 6.0 SCIENCE AND EVERYDAY PHENOMENA
Lecture 13: Foams and Bubbles
| Prev | Next | Search | Syllabus | Selected References | Home |
I've always liked coffee, but my delight took on a new dimension when I started drinking
espresso and cappuccino. My spirits lift every morning as I grind and measure the coffee,
put it into the espresso machine, and watch it drip. Then comes the best part: I plunge
the steam pipe into milk, making bubbles which cling closely to each other to form the
magical froth that turns an espresso into a cappuccino. Sometimes the bubbles are tiny
and densely packed, which makes the foam stiff and upstanding. Sometimes they're large
and fragile, forming a shaky structure that soon collapses. The variations depend mostly
on the type and brand of milk, and the pressure of the steam.
Sidney Perkowitz
Universal Foam: From Cappuccino to the Cosmos
Walker & Co., NY 2000, p. 1.
Topics
-

The Great Wave of Nanagawa. Hokusai Katsushika (1760 - 1849)
Perkowitz's book is clearly and simply written, and provides one of the best introductions to this relatively new field. You can find Chapter 1, Bubbles
and Geometry on line. Here is a concise statement about definitions, examples, and
outstanding problems:
Bubbles
and Geometry on line. Here is a concise statement about definitions, examples, and
outstanding problems:
"For all its frothiness, foam is a surprisingly intricate formation that has impact on astronomy, biology, chemistry, physics, and mathematics. Foam is not exclusively a solid, liquid, or gas; it is made of bubbles or cells of gas within a liquid or a solid, and it combines characteristics of all three states of matter [ … ] Grasping the complexities of foam and its bubbles requires scientific tools and knowledge that have come along only recently [ … ] At the planetary scale, there is the foam of ocean whitecaps that covers millions of square miles and influences the world's climate. Pumice, a type of foamy rock emitted from volcanoes, carries clues to the geologic history of the Earth. At the cosmic scale, the billions of galaxies making up the universe are arranged as if they lay on the surface of immense bubbles within a gargantuan foam [ … ] Although much is known about foam, it is studded with scientific mysteries. Some represent unexplained properties of foam seen even in everyday use, such as the flowing behavior of whipped cream, a singular combination of solid and liquid characteristics. Others arise in the laboratory, such as the extraordinary effect called sonoluminescence, where a bubble floating in liquid changes sound into light. " [ ibidem, p. 2 - 3 ]
Foams are indeed to be found just about everywhere, from contraceptives to shaving cream, from foods (bread and chocolate, whipped cream, beer, champagne and other carbonated drinks) to detergents (domestic and industrial soapsuds); from fire-extinguishing compounds to ore mining and processing; from manufacturing and construction processes (metallic foams) to decontamination technologies (chemical foams to sequester and neutralize toxic chemicals); etc. -
It is possible to begin to study foams with as simple a setup as "a transparent plastic bottle
[ … ] half filled with ordinary tap water and a dash of liquid soap [ … ]
simply shake the bottle. The clear liquid turns into a mass of bubbles, each surrounded by soapy water
[ … ] Omit the soap, and bubbles still form [ … ] but they die the
instant you stop shaking [ … ] As you shake, you see a radical transformation.
transparent air and water are transmuted into an opaque white mass [ … ] A close look
through a magnifying glass shows that the foam contains bubbles of various sizes [ … ]
They are piled like cannonballs on a courthouse lawn, but in a disorderly heap rather than a perfect
stack. Each is a sphere, surrounded by liquid that isolates it from its neighbors, This is a wet
foam, meaning it is more liquid than gas [ … ]
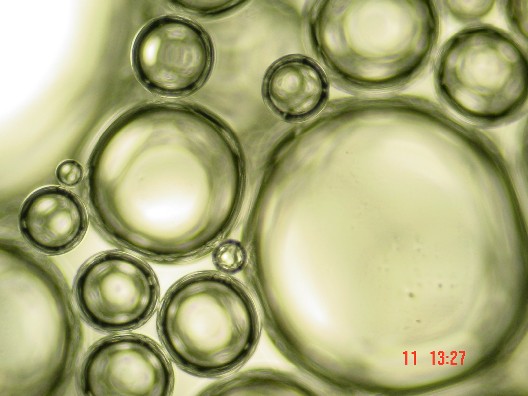
Bubbles in Wet Foam
[ … ] If you put the bottle down, and wait, the foam changes. Slowly, the proportion of liquid decreases as the water between bubbles drains downward under the pull of gravity [ … ] As the drainage continues, the wet foam becomes something more complex: a dry foam, with more gas than liquid. The walls between adjoining bubbles become very thin [ … ] The shape of the bubbles also changes. Instead of spheres, adjoining bubbles distort each other into polyhedrons, three dimensional bodies with flat or gently curved faces where they abut. Each resembles a soccer ball or a geodesic dome, except that its facets are random in size, shape and orientation. Only after several days does the foam finally die, as the last few polyhedral cells vanish, leaving the bottle as it began, half full of soapy water. " [ ibidem, p. 10 - 11 ]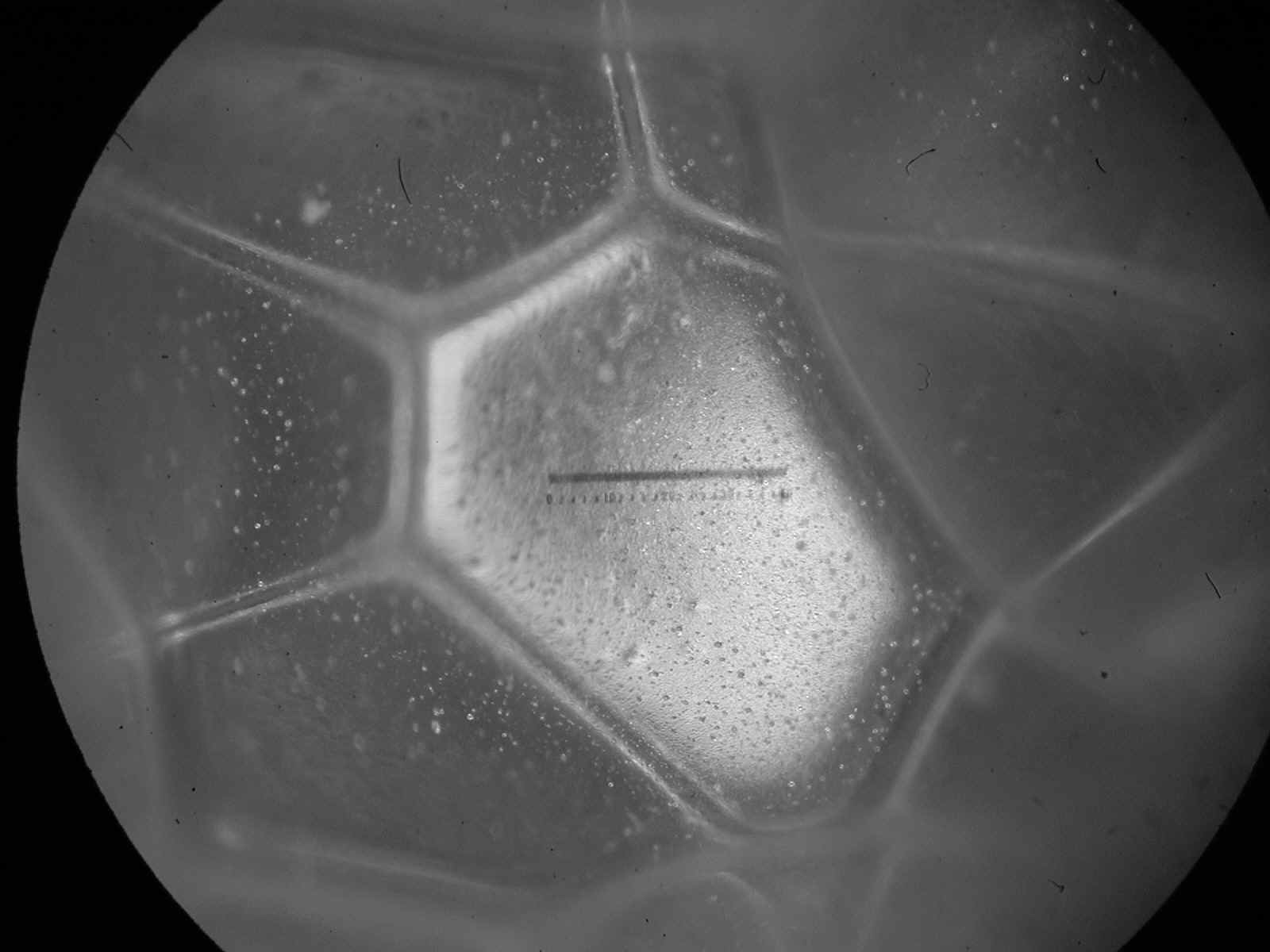
Polyhedral Bubbles in Polyurethane Foam
As you can see, the structure of foams is very complicated, yet such structure must be understood if we want to understand the dynamical properties of foams. One of the pioneers in this area of research was the Belgian physicist Joseph Antoine Ferdinand Plateau (1801 - 1883). He experimented with "a solution of soapy water and glycerine and dipped wire contours into it, noting that the surfaces formed were minimal surfaces." This is a beautiful example of analog computing. The problem, known since as the Plateau Problem, "is to show the existence of a surface of minimal area with a given boundary curve." [ ibidem ] It is a very hard problem, and was only solved by Jesse Douglad and Tibor Radó in the 1930s. The basic idea is that the surface tension of the solution forces the bubble into the shape that has the least surface area. When detached from the wireframe, such shape is a sphere. As to surface tension, here is a nice definition: "The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface." [ from Hyperphysics ] The role of soap, glycerine, etc. is that of a surfactant, namely of an agent which reduces the surface tension, allowing water, for example, to be wetter, i.e. to spread over and wet surfaces more easily. If you think that this kind of research is purely academic (or just plain entertaining), consider this: "It [is] possible to use soap films to answer difficult mathematical questions called minimal area problems. These arise in mechanics, optics, and the theory of relativity [ … ] If you want to build a highway linking a certain number of towns, what configuration requires the least length of road [ … ] while ensuring that one can drive between any pair of towns? [ … ] a soap film automatically indicates the correct route when it clings to a small scale model giving the location of the towns." [ Perkowitz, op. cit., p. 18 ]. -
If wet foams are relatively easy to study, dry foams are much, much harder.
"Each bubble within a foam must take a shape that gives minimal surface area and must also be consistent with the constraining presence of its neighbors. If the bubbles are isolated, as in a wet foam, that shape is a sphere. But in a dry foam, where only thin films separate the bubbles, they must fit together to completely fill the volume of the foam, which cannot be done with spheres. A more complex shape is needed, which turns out to be hard to define mathematically even for a foam simplified and idealized, with all the bubbles the same size. This particular pattern of nature is a long-standing enigma whose solution is still elusive."
The history of the many attempts to solve this problem probably starts with William Thomson (Lord Kelvin) (1824 - 1907), who "in 1877, drawing on shapes known for iron crystals [ … ] theorized that the bubble with minimum area was a strange-looking figure with six square and eight hexagonal faces [ … ] called tetrakaidecahedron [ … or ] Kelvin's bedsprings."
[ Perkowitz, op. cit., p. 19 ].
[ Perkowitz, op. cit., p. 19 - 20 ].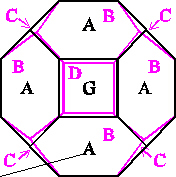
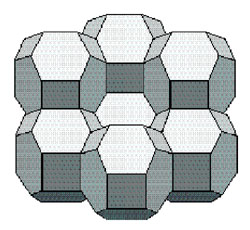
Model and Cluster of Kelvin Tetrakaidecahedra
"For over a century, Kelvin's solution was the undisputed champion; all that was missing was a proof. The proof did not materialize, however, and in 1994 Weaire and Phelan finally explained why: Kelvin's partition is not the best way to fill space." You can find this statement in a very good article by Erica G Klarreich entitled Foams and Honeycombs, which appeared in the March-April 2000 issue
of American Scientist. The search for a solution is not over yet.
Foams and Honeycombs, which appeared in the March-April 2000 issue
of American Scientist. The search for a solution is not over yet.
-
No matter how familiar bubbles may appear to us, they have also enabled us to probe the subatomic structure of
matter. The components of the subatomic world are too small to be seen, no matter which magnifying device
we choose. In addition, because they are fully subject to the quantum mechanical laws, even if we had sufficient
resolving power we would not be able to see them. At best they would appear as fuzzy images.
Around 1955, D Glaser invented a device, named the Bubble Chamber,
which provided "the aesthetically most appealing visualization of subnuclear collisions." A bubble chamber
consists of a container full of a pure liquid such as hydrogen, propane, freon, etc., which is "heated above its
boiling point, [and] which is then suddenly expanded, starting boiling where passing charged particles
have ionized the atoms of the liquid." The container is placed on the path of a particle beam, and is surrounded
by heavy magnets. The trail of bubbles left by a particle crossing the interior of the chamber can be photographed.
Below is one such photograph, which captured the famous discovery of a new particle, the Ω–.

The Discovery of the Ω–
Readings, Resources and Questions
- For a gentle introduction to soap bubbles, visit Ron Hipschman's Bubbles, one of the exhibits at the Exploratorium, The Museum of Science, Art and Human Perception.
- Why Does Hydrogen Peroxide Foam When You Put It On A Cut?.
- In a foam, bubbles come in all sizes, a fact which in many applications is not that desirable. Read Bursting the Bubble Barrier, which reports a successful and rather simple new technique for creating foams with identical bubbles.
- Antibubbles are "skins of air which float around underwater, and vanish when touched !"
- Foams and Bubbles features very interesting images and short films of experiments with wet foams.
-
"Light and fluffy foam is actually serious business. To ensure that canned draughts have predictable,
lasting heads, for example, Guinness developed the Widget, a plastic ring that releases nitrogen into the beer
as it is opened; it won the Queens Award for Technological Advancement in 1991. Coca-Cola, on the other hand,
has sought ways to create more fleeting foams from soda fountains. And all the while, physicists have chased
after a theory to explain how foam behaves. Now they may have one, reported in this week's issue of Physical
Review Letters by Howard Stone and his colleagues at Harvard University." Read this short but interesting
article which appeared in the May 16, 2001 issue of Scientific American: New Theory Explains the Physics of Foam
Here are references to everything you always wanted to know about beer, champagne and other foamy beverages: Beer Foam Physics,
Chemical Fizzics,
Richard Zare Explains the 'Fizzics' of Beer,
The Physics of Fizz,
Champagne Bubble Mystery Uncorked.
Here are a few questions to get you thinking about champagne and its bubbles [ from Chemical Fizzics: A Touch of the Bubbly ]
- "Why are bubbles spherical in shape?
- Why can you easily make bubbles with soapy water but not with pure water?
- hy do bubbles form at interfaces (cracks on the glass walls) but not in the pure liquid?
- Why do bubbles in a carbonated beverage rise, and why do they accelerate in rise rate and grow in size as they rise?
- What happens when two or more bubbles collide?
- What controls the height of foam in a carbonated beverage as a function of time?
- Why does a bottle of carbonated beverage that has been agitated before opening seem to explode whereas one that has sat still is well behaved?
- Why do you get more lather in hot water than in cold water?
- Why do bubbles fall through the air at a constant rate?
- Why is the foaming of a carbonated beverage poured over ice the strongest the first time but less strong subsequent times?"
- From the kitchen sink to the Space Station, or Strange Foam. "The physics underlying common everyday foams is poorly understood. An experiment scheduled to fly on the International Space Station will help fill in the gaps."
- Finally, three good references with a rather extensive coverage of foams: Foam Physics at Trinity College, Dublin, Frequently Asked Questions in Colloid and Interface Science, and Dennin Research Group. Sample Talks and Posters. On the last site, please click on one or both of the items under "Distinguished Assistant Professor Award for Research." These are the pdf versions of a very good talk (with pictures) entitled Foams and Stripes.
Picture Credits: Rutgers U · U of Twente · UCLA
U of Warsaw · Industrial Heating · CERN
Last Modification Date: 24 November 2005