Instructions
Problem: You submitted a sample with the expected formula of C20H23N5 for microanalysis. However, the values found differ from the calculated ones. You input the formula and microanalyses values, like this:
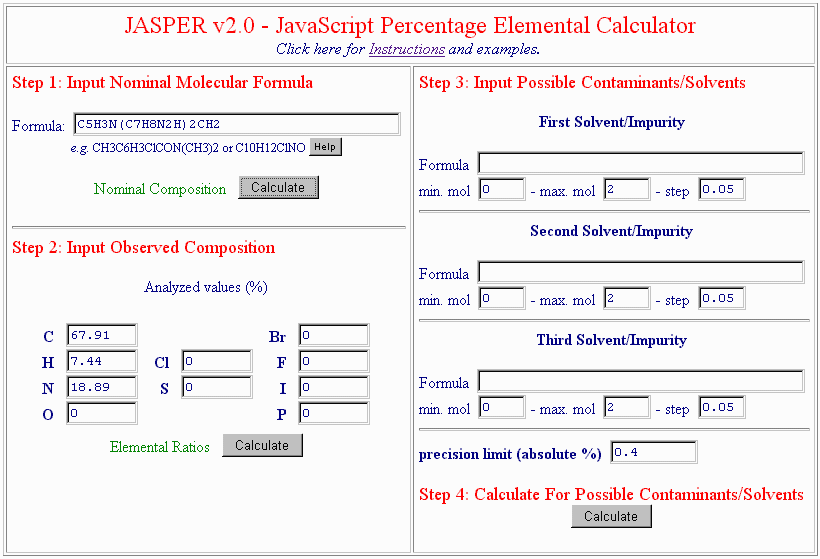
then press the "Calculate" button next to Nominal Composition and obtain the following result:
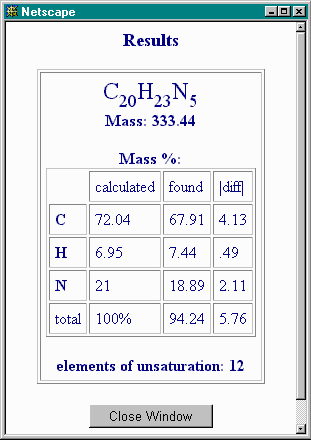
This tells you that there are severe discrepancies in %C and %N and nearly 6% of the mass not accounted for by C, H and N. You close this window and, for some insight, you press the "Calculate" button next to Elemental Ratios and obtain the following result:
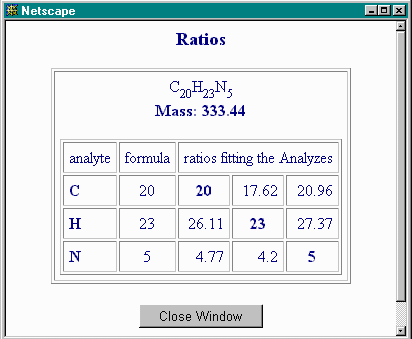
This tells you that, relative to N, there is excess C and H over and above what the nominal formula provides.
You can now guess what impurity or solvent caused this difference by inputting up to 3 different molecular formulas of solvents or potential impurities. Many times a good guess is:
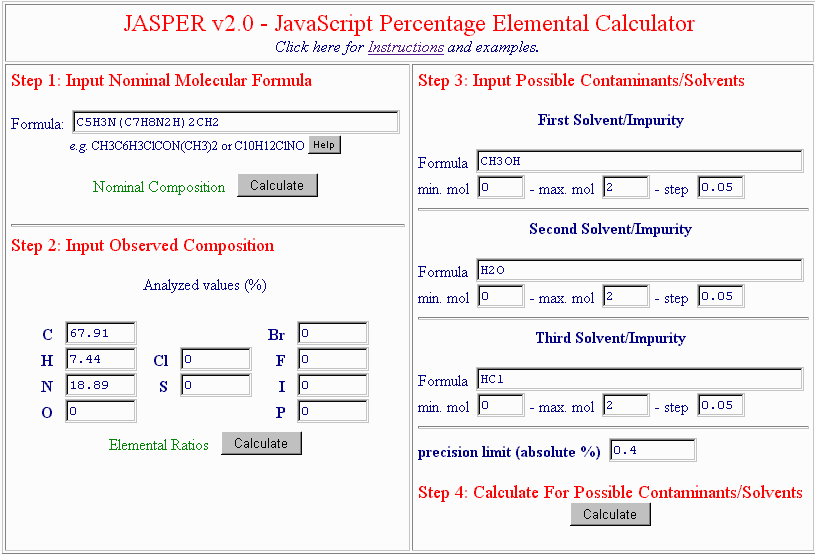
For the moment, leave the permissible equivalents and the step size at their default values. After a few moments of calculation, this is the result:
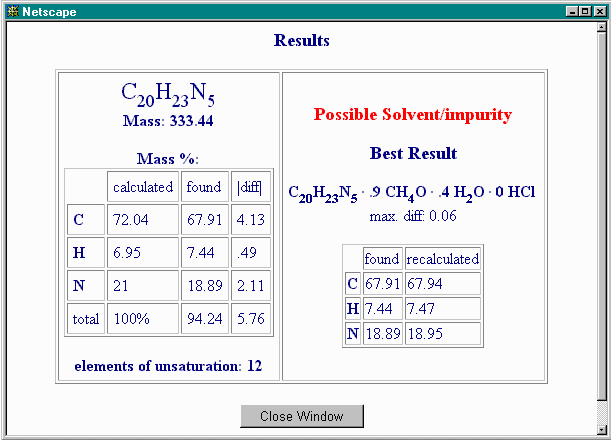
Hence, both methanol and water are likely contaminants, but HCl seems to be absent. In other words, all non-C, non-H and non-N content can be accounted for by oxygen from methanol and water. Nevertheless, it's possible that HCl and not oxygen-containing materials accounts for the 5.76% non-C, non-H, non-N component. If both methanol and water are removed as possible contaminants, either by deleting the formulae or by putting 0 ("zero") in the corresponding "max. mol" box, like this:
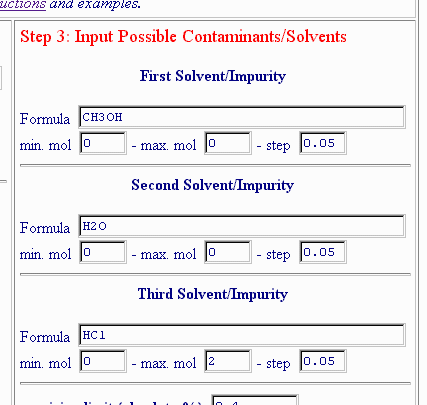
The result is:
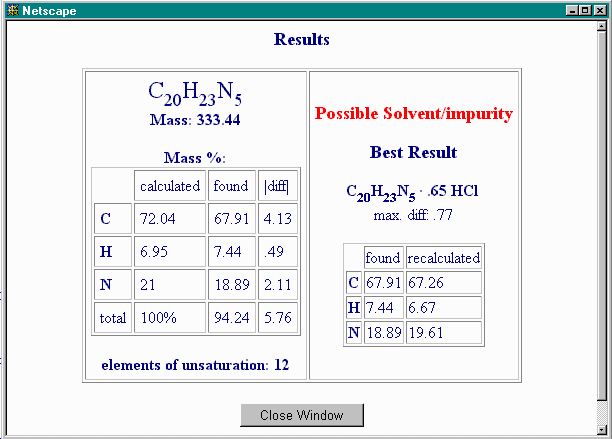
This is less convincing since the excess of C over N is not accounted for, and the maximal difference between calculated and observed analyses is much higher.
You next wonder if, instead of 0.9 mol of CH3OH, there could really be a full solvate. You add "*CH3OH" to the nominal formula, like this:
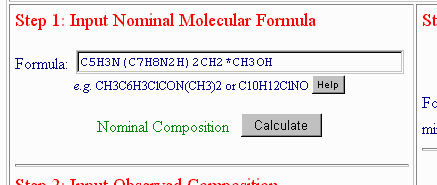
and recalculate the Nominal Composition, to obtain:
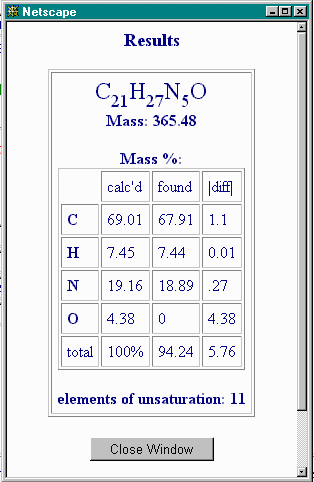
The calculated %O accounts for much of the non-C, non-H, non-N content, but not all of it. You then allow water as a contaminant by restoring the appropriate "max. mol" value to 2 (say), and this gives:
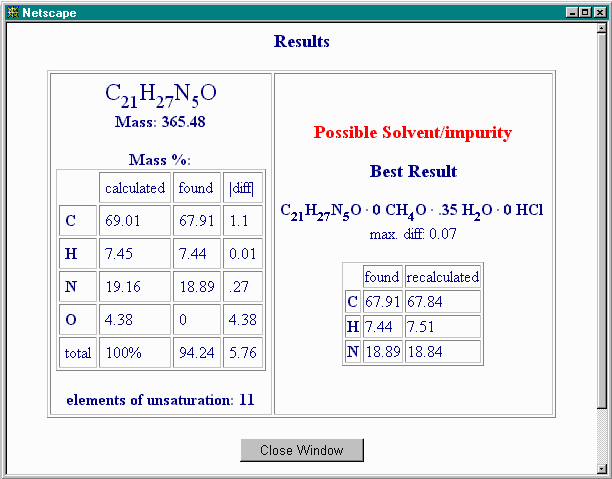
Indeed, some small amounts of water may be present alongside a possible methanol solvate.
Just to be safe, you check whether or not the contaminant might be a carbonate salt of the amine (from absorption of atmospheric CO2 and water). You remove the "*CH3OH" from the nominal formula and input CO2 and H2O as possible contaminants, on the right-hand side as before. It turns out that this gives a much less satisfactory result, as follows:

You conclude that either the sample was improperly dried of solvents or that it crystallizes as the methanol solvate which happens to be wet. You verify this by taking an NMR spectrum of the same material that you had submitted and this shows that, indeed, there is approximately one equivalent of methanol present. Since the presence of water cannot be ascertained by NMR, you dry a new sample under high vaccuum and take the NMR spectrum again. If the presence of methanol is re-confirmed, then you have a true solvate. You re-submit this new sample and, because you suspect it to be hygroscopic, you instruct the analyser to dry the sample before analysis.