Self-assembled organic materials have recently drawn significant attention in the context of efficient energy, charge and ionic transport pro- cesses.[1] In the context of these self-assembly processes, phosphorus has proven to be a very versatile main group element beyond purely hydrocarbon-based materials. As an enzyme mimic, for example, phosphorus can act as coordinating center to form macrocycles and cages in which catalytic activities and energy transfer could occur.[2] Another inspiration for materials chemistry are phospholipids, a major component of cell membranes; researchers are now successfully copying and utilizing the amphiphilic character of phospho-lipid-analogues in self-assembled materials.[3]
Our group and others, have recently established phosphole-based pi-conjugated systems as an intriguing new class of electronic materials with a variety of unique and versatile properties.[4] Within this stream of our research, we have started to further tailor the phosphole system by installing self-assembly properties and a investigating the energy and ionic transport properties of assemblies.[5,6] Our research in this area uses phosphaorganic conjugated scaffolds toward the self-assembly properties of the novel systems that we have termed "phosphole-lipids". To date we have accessed highly ordered liquid crystals as well as organogels based on these phosphole-lipids.[6,7] We were able to furthermore highlight their ability to noticeably respond toward external stimuli. The basic structure (phosphorus chemistry) - property (nano-/micro-structure and energy transfer) relationship is currently being investigated and modeled toward further developing this intriguing class of compounds for a variety of potential applications in organic electronics.
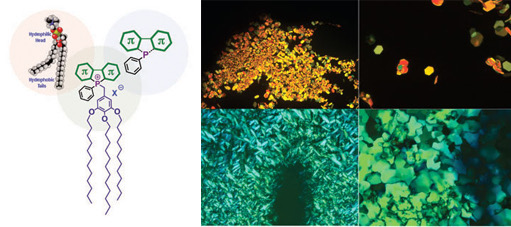
General structure of a phosphole-lipid (left) and corresponding soft crystal phases (right)
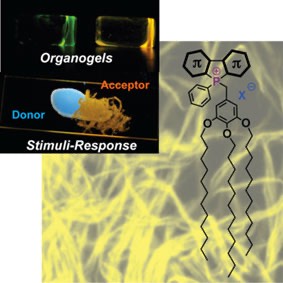
Phosphole-lipid-based organogel fibres (bottom right) and mechanochromic behaviour upon scratching (top left)
Further Reading:
[1] (a) Kato, T.; Mizoshita, N.; Kishimoto, K. Angew. Chem. Int. Ed. 2006, 45, 38. (b) Kato, T. Angew. Chem. Int. Ed. 2010, 49, 7847. (c) Zhang, L.; Che, Y.; Moore, J. S. Acc. Chem. Res. 2008, 41, 1596.
[2] Wiester, M. J.; Ulmann, P. A.; Mirkin, C. A. Angew. Chem. Int. Ed. 2011, 50, 114.
[3] Shimizu, T.; Masuda, M.; Minamikawa, H. Chem. Rev. 2005, 105, 1401.
[4] Baumgartner, T; Réau, R. Chem. Rev. 2006, 106, 468.
[5] Ren, Y.; Baumgartner, T. J. Am. Chem. Soc. 2011, 133, 1328.
[6] Ren, Y.; Kan, W. H.; Henderson, M. A.; Bomben, P. G.; Berlinguette, C. P.; Thangadurai, V.; Baumgartner, T. J. Am. Chem. Soc. 2011, 133, 17014.
[7] Ren, Y.; Kan, W. H.; Thangadurai, V.; Baumgartner, T., Angew. Chem. Int. Ed. 2012, 51, 3964.