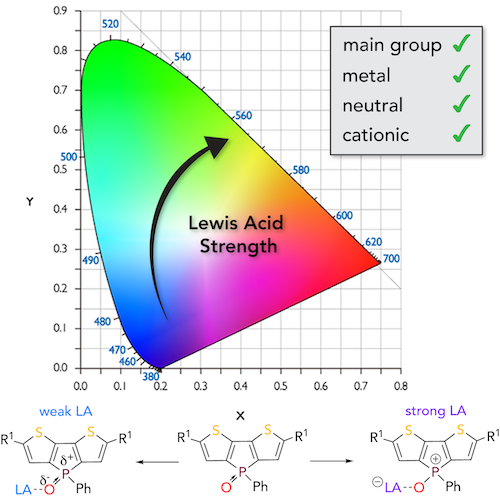
The sensing of Lewis acids, and their strength in particular, has recently moved into the focus of many researchers due to the rapidly rising importance of Lewis acids as catalysts for chemical transformations and in materials science.[1,2] However, the inherent structural and chemical diversity of Lewis acids in general poses a considerable challenge with regard to a unifying strategy. While several methods have been established that date back to the 1960’s, none of them is without flaws. In collaboration with the Caputo group at York University, we have developed a novel fluorescence-based method that we termed Fluorescent Lewis Adducts (FLA). The FLA method provides a groundbreaking solution to these problems with considerable impact for many different areas. It is very sensitive, independent of the Lewis base and applicable to most different types of Lewis acids.[3] Neutral, charged, main group-, and transition metal-based species have been quantified for the first time and we have been able to expand this to over 50 Lewis acids.[4] We are currently elaborating the link between the Lewis acid unit, LAU (obtained from the FLA method), with the respective chemical reactivity as catalyst, where initial findings that also consider the impact of solvent, are extremely promising.
Further reading:
[1] H. Yamamoto, Lewis Acids in Organic Synthesis, Wiley-VCH, 2008.
[2] T. Baumgartner and F. Jäkle, Main Group Strategies towards Functional Hybrid Materials, John Wiley & Sons Ltd., 2017.
[3] J. R. Gaffen, J. N. Bentley, L. C. Torres, C. Chu, T. Baumgartner, C. B. Caputo, Chem 2019, 5, 1567.
[4] J. N. Bentley, S. A. Elgadi, J. R. Gaffen, P. Demay-Drouhard, T. Baumgartner, C. B. Caputo, Organometallics 2020, 39, 3645.