Neural mechanisms for predictive head movement strategies during sequential gaze shifts
- Jachin A. Monteon1,2,
- Marie Avillac1,3,
- Xiaogang Yan1,2,
- Hongying Wang1,2, and
- J. Douglas Crawford1,2,4
+ Author Affiliations
- Address for reprint requests and other correspondence: J. D. Crawford, York Centre for Vision Research, Lassonde Bldg., York Univ., 4700 Keele St., Toronto, ON, Canada M3J 1P3 (e-mail: jdc@yorku.ca).
- Submitted 14 March 2012.
- Accepted 24 August 2012.
Abstract
Humans adopt very different head movement strategies for different gaze behaviors, for example, when playing sports versus watching sports on television. Such strategy switching appears to depend on both context and expectation of future gaze positions. Here, we explored the neural mechanisms for such behaviors by training three monkeys to make head-unrestrained gaze shifts toward eccentric radial targets. A randomized color cue provided predictive information about whether that target would be followed by either a return gaze shift to center or another, more eccentric gaze shift, but otherwise animals were allowed to develop their own eye-head coordination strategy. In the first two animals we then stimulated the frontal eye fields (FEF) in conjunction with the color cue, and in the third animal we recorded from neurons in the superior colliculus (SC). Our results show that 1) monkeys can optimize eye-head coordination strategies from trial to trial, based on learned associations between color cues and future gaze sequences, 2) these cue-dependent coordination strategies were preserved in gaze saccades evoked during electrical stimulation of the FEF, and 3) two types of SC responses (the saccade burst and a more prolonged response related to head movement) modulated with these cue-dependent strategies, although only one (the saccade burst) varied in a predictive fashion. These data show that from one moment to the next, the brain can use contextual sensory cues to set up internal “coordination states” that convert fixed cortical gaze commands into the brain stem signals required for predictive head motion.
actions must be appropriately coordinated to suit moment-to-moment circumstances, e.g., related to experience (Bichot et al. 1996), environment (Watanabe and Sakagami 2007), and expectation of future events (Oommen et al. 2004). Take, for example, coordinated eye-head gaze shifts (e.g., Bizzi et al. 1972; Gandhi and Katnani 2011; Guitton 1992). Head movements can behave in a predictive fashion not only for the immediate next gaze shift (Corneil and Munoz 1999) but also for anticipated future gaze shifts. In situations in which the expected range of gaze positions is limited and recentering is predictable, as in watching television or reading, head motion is minimized (Proudlock et al. 2003). In activities in which the gaze range is unlimited and one cannot predict the next point of interest, as in sports or driving, the head moves more with gaze to keep the eyes centered and ready (Land 1992). However, very little is known about the computational and neural implementations of such strategies.
Two computational strategies could account for context-dependent changes in eye-head coordination, depending on the nature of the task. First, it could be that repetition of behavior in the same conditions leads to optimization over a series of trials (Everling and DeSouza 2005). This has already been demonstrated: humans moved their head more when they repetitively produced a series of two centrifugal gaze shifts (Out-Out), compared with repetitive series of Out-In gaze shifts, i.e., outward and back to center again (Oommen et al. 2004). In other situations primates might learn to associate a specific set of perceptual cues with the optimal motor coordination strategy. This option has the advantage that (once each such pairing is learned) it could produce optimal behavior in the very first response, without the need for repetition. It is well known that the brain is capable of optimizing motor output for context (e.g., Shelhamer and Clendaniel 2002; Wainscott et al. 2005), but the moment-to-moment influence of visual cues on eye-head coordination during gaze shifts has not been explicitly studied.
Thus the first goal of the present study was to determine whether primates can learn to associate visual cues with future gaze positions, and thus optimize their eye-head coordination strategies on a moment-to-moment basis. To do this, we modified the paradigm used by Oommen et al. (2004) so that the two types of gaze sequence (Out-In vs. Out-Out) were instead randomly intermingled and always paired with the two consistent predictive visual cues.
The second goal of this study was to determine the role of cortical gaze commands in these adaptive control strategies. An obvious place to examine this question is the frontal eye field (FEF). The FEF provides the major cortical gaze control output through projections to both the superior colliculus (SC) (Komatsu and Suzuki 1985; Sommer and Wurtz 2000) and brain stem premotor circuitry (King et al. 1980; Segraves 1992; Sparks and Hartwich-Young 1989). The FEF is central for target selection (Schall et al. 1995; Thompson et al. 1997; Trageser et al. 2008) and in the visuomotor transformations for gaze control (Schall 1997). The FEF is also known to participate in context-dependent behaviors (Bichot et al. 1996; Everling and Munoz 2000; Gold and Shadlen 2000). Unilateral ablation of the FEF in rodents causes asymmetries in ocular nystagmus (Bähring et al. 1994), whereas electrical microstimulation of the FEF evokes coordinated eye-head gaze shifts in head-unrestrained animals (Bruce et al. 1985; Chen 2006; Knight and Fuchs 2007; Monteon et al. 2010; Tu and Keating 2000), although some nearby sites produced isolated or torsional head motion (Chen 2006; Monteon et al. 2010). Even “subthreshold” FEF stimulation modulates neck muscle activity (Corneil et al. 2010). Taken together, these findings suggest that the FEF could play a role in predicting future gaze sequences and choosing adaptive eye-head coordination patterns.
How might the FEF influence the optimal pattern of head contribution, according to visual context, for future sequences of gaze shifts? It could be that cue-dependent modulations in head contribution require moment-to-moment variations in cortical output to the brain stem, meaning that different FEF outputs are required to produce different coordination strategies. Alternatively, it could be that perceived changes in contextual information trigger learned “coordination states” at some point further downstream (MacDonald et al. 2000; Miller and Cohen 2001; Wise and Kurata 1989) that are then accessible to a fixed cortical output. For example, a visual cue could trigger a pattern of neuromuscular excitability downstream from the FEF, involving increased neural excitability and/or background activation of muscle groups (Corneil et al. 2008). One way to test between these possibilities is to electrically stimulate the FEF to produce a fixed cortical activation and then observe whether the resulting eye-head coordination patterns depend on visual cues in the same way as behavior.
The third goal of our study was to determine whether context-dependent signals related to future gaze positions and optimal eye-head coordination can be detected in the downstream targets of the FEF. A logical place to look for this is the SC, which receives FEF input (see above) and plays a prominent role in eye + head gaze control (Gandhi and Katnani 2011; Guitton 1992). Electrical microstimulation of the SC in head-unrestrained animals is generally thought to produce fixed gaze shifts in retinal coordinates (Klier et al. 2001) with normal patterns of eye-head coordination (Freedman et al. 1996; Klier et al. 2003). Likewise, most studies that have recorded SC activity during head-unrestrained gaze shifts have identified gaze (Freedman and Sparks 1997a; Munoz et al. 1991)- or target (DeSouza et al. 2011)-related signals. Other studies have claimed evidence for separate eye-head control in the SC (Cowie and Robinson 1994). In particular, head position modulates SC activity (Nagy and Corneil 2010), and there is recent evidence for head movement-related activity within the deep layers of the SC (Rezvani and Corneil 2008; Walton et al. 2007). Modifications of context-dependent adaptation of head movement were not preserved during stimulation of the SC (Constantin et al. 2004), suggesting that other signals are required for the head strategy. However, those modifications were induced by physical limitations on the visual range, not through self-selected strategies. Currently it is not known whether the SC possesses neural signals related to such adaptive head control strategies. Such signals could be effector (i.e., eye vs. head)-related, or they could simply be modulations on the gaze-burst that could trigger different downstream responses.
In summary, our aim was to determine if and how monkeys utilize context-dependent, predictive gaze control strategies. We did this by combining three related experiments: 1) behavioral training and analysis, 2) FEF microstimulation, and 3) single-unit recording in the SC. Specifically, we trained three monkeys to associate color cues with two different sequences of future gaze position: either two centrifugal movements or a centrifugal movement followed by a centripetal return movement. We hypothesized that monkeys would modify head control strategy for a current gaze shift to suit the subsequent gaze shift. When such behavioral modifications were observed, we then investigated their neural mechanisms. We previously reported that stimulation of the FEF in two of these animals produced naturally coordinated gaze shifts (Monteon et al. 2010). Here, we stimulated similar FEF sites, in conjunction with a modified version of our new cue-dependent task, to see whether the adaptations observed in behavior were preserved during a fixed FEF output. Finally, we sought evidence for cue-dependent neural signals further downstream by recording from task-related SC neurons in our third animal. In brief, we found that 1) monkeys do develop cue-dependent strategies for predictive eye-head coordination, 2) these strategies are preserved during FEF stimulation, and 3) the SC possesses two types of perisaccadic signals that correlate to these behavioral strategies.
MATERIALS AND METHODS
General
Experiments were performed in three alert behaving female rhesus monkeys (Macaca mulatta), animals M1, M2, and M3, weighing 5.3, 6.5, and 6.5 kg at the beginning of the experiment. Experiments were done in accordance with Canadian Council on Animal Care guidelines and were preapproved by the York University Animal Care Committee. Under general anesthesia and with sterile procedure, a chamber (20-mm diameter) was implanted over the right (M1) and right/left (M2) arcuate sulcus (Monteon et al. 2010; Robinson and Fuchs 1969) (stereotaxic coordinates A 22 L 18–20) and over the SC (A 05 L 00) in M3. Scleral search coils were surgically implanted into one eye of each animal to monitor eye position. In addition to the coils implanted in the eyes, two coils were fastened onto a plastic platform on the skullcap to record head position in each animal (Crawford and Vilis 1991; Tweed et al. 1990).
During experiments, monkeys wore a primate jacket and sat in a modified primate chair such that the head and neck were free to move as desired. The upper body (to the shoulders) was prevented from rotating in the yaw direction by the use of plastic molding and restraints that attached the primate jacket to the chair. Animals M1 and M2 sat in front of a hemispherical screen having a radius of 100 cm. For these animals, targets were 3-mm-diameter LEDs affixed to the back of the screen at various eccentricities. Eight LEDs in the array were positioned at 20° (M1 and M3) or 25° (M2) distance from a central LED, in radial directions of 0, 45°, 90°, 135°, 180°, 225°, 270°, and 315°. For M1 and M3 the remaining eight LEDs were positioned at 50° distance from the central LED in the same radial directions as the previous set of LEDs. For M2 six LEDs were positioned at 50° distance from the center, while the other two LEDs (in radial directions 0 and 180°) were positioned at 55°. Slightly more eccentric targets were used in M2 because this animal tended to move its head less than the other animals. Animal M3 sat in front of a large flat translucent screen, and a NEC WT600 DLP projector was used to back-project 0.5° target images at 20° and 50° eccentricity in the same eight directions.
Behavioral Task and Training
In our two basic paradigms, monkeys were trained to make centrifugal/centripetal (Out-In) and centrifugal-centrifugal (Out-Out) sequences of gaze shifts and were provided with a color cue that predicted the location of the second target. All trials started with this cue presented at the center of the screen, which also served as the first fixation point. Monkeys were trained to perform by operant conditioning for liquid reinforcement, and training was only done with the head unrestrained.
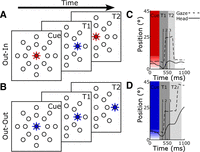
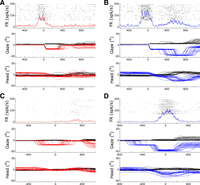
During the Out-In paradigm (Fig. 1A), a red cue was lit at the center of the screen and subjects were required to maintain fixation for 400 ms (M1), 500 ms (M2), or 1,000 ms (M3; the duration was prolonged for the last animal to establish stable baseline neural firing rates in the unit recording experiment described below). Animals had to fixate the cue within a spatial window that ranged from 5° to 7°. At the end of the fixation period the cue was extinguished, and at the same time a peripheral target (T1) was illuminated. The distance of T1 from the central cue was 20° for M1 and M3 and 25° for M2. Animals had to fixate T1 for at least 60 ms (90 ms in M3) within a spatial window that ranged from 8° to 12°. Then, after an additional 58.3-ms lag related to electronic processing, T1 was extinguished and a central target (T2) was lit immediately afterwards. Factoring in behavioral reaction times, this gave a total fixation duration in the range of 253.3 ± 1.2 ms. Animals were then required to saccade toward and fixate T2 for at least 60 ms within a spatial window of 10° to 13.5°. The sequence of behavioral events for a typical trial is shown in Fig. 1C, and more examples are illustrated in Fig. 9, A and C.
Behavioral paradigms. A and B: sequence of external events. Colored symbols indicate the LED/projected gaze target at each interval; the room was otherwise dark. At the beginning of each trial monkeys fixated at a central color cue for 400 ms (M1) 500 ms (M2), or 1,000 ms (M3). A: Out-In paradigm; the central cue was red. When the cue was extinguished, a peripheral target (T1) appeared in 1 of 8 directions and animals fixated at it for 60 ms; then the central target (T2) reappeared. B: Out-Out paradigm; the central cue was green (represented here as blue). When the cue was extinguished, a peripheral target (T1) appeared in 1 of 8 directions and animals fixated at it for 60 ms. Finally, a more eccentric target (T2) appeared along the same radial line. C and D: sequence of events illustrated with data from 2 typical trials. Horizontal gaze and head positions are plotted as a function of time. These trajectories belong to monkey M1 and were recorded on the same day and target direction. Colored areas depict the time in which the color cue was shown (red cue for Out-In, blue for Out-Out). C: Out-In paradigm. D: Out-Out paradigm. Numbered vertical lines represent the 3 time epochs used to analyze the data: 1, the beginning of gaze or head movements (whichever was first); 2, the end of the gaze movement; and 3, the end of the head movement (see materials and methods for details).
The Out-Out paradigm (Fig. 1B) was identical except for two aspects: first, the central cue was green in color, and second, target T2 was not positioned at the center of the screen; instead it was positioned in a more eccentric position than T1, which was 50° from the center of the screen for M1 and M3 and 50° or 55° for M2. After fixation at this eccentric T2 for several hundred milliseconds, animals returned to the center target. The sequence of behavioral events for a typical data trial is shown in Fig. 1D, and more examples are illustrated in Fig. 9, B and D.
Note that, visually, the two paradigms were identical up until the presentation of the second target, except for the color of cue. All eight directions were used for training in all animals with both paradigms and for experimental recording in animals M1 and M2. In animal M3 data recorded for the behavioral analysis were matched to SC neural properties recorded simultaneously, as described below. M1, M2, and M3 were trained like this for 10, 12, and 12 wk, respectively, before data were collected. During experiments, the reaction times for the first gaze shift varied from 238 to 310 ms and reaction times for the second gaze shift ranged from 390 to 430 ms.
Stimulation Paradigm
This experiment was performed in animals M1 and M2 and was randomly interleaved with trials from the Out-In and Out-Out paradigms. The intent here was to unexpectedly replace the first gaze shift from the behavioral paradigm with a gaze shift evoked through stimulation of the FEF. To do this, the Out-In and Out-Out paradigms were modified to create the Stim-In and Stim-Out paradigms.
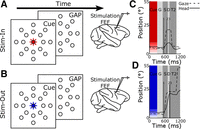
In the Stim-In paradigm (see Fig. 4A) a red cue was lit at the center of the screen and subjects were required to maintain fixation for 300 ms (M1) and 450 ms (M2). Animals had to acquire fixation of the cue within a fixation window that ranged from 5° to 7° and a reaction time of 1 s. After this period the cue was extinguished and a gap period was introduced, during which monkeys had to maintain fixation to the location where the cue was presented. This gap duration was 210 ms for M1 and 340 ms for M2. After the gap, a train of electrical pulses (300 Hz, 60 pulses, 200 ms, 60–90 μA) was delivered on the right or left FEF (Chen 2006). After this, a delay period was introduced in order to allow enough time for the completion of any evoked eye movements (see Fig. 4C, light gray area labeled “D”), i.e., prevent the presentation of T2 before the evoked movements were completed. Larger movements from a given FEF site take a longer time to complete compared with smaller movements evoked from a different site. Thus before each experiment we did an online visual inspection of the evoked movements and then adjusted the delay period accordingly (range 70–300 ms). This delay was then held constant for a given brain site, regardless of the paradigm. After the delay, animals were required to fixate a central target (T2) for 60 ms (Fig. 4, A and B).
The Stim-Out paradigm (see Fig. 4B) was identical to the Stim-In paradigm except for two aspects: first, the central cue was green in color (shown here as blue), and second, target T2 was not positioned at the center of the screen; instead it was positioned in an eccentric position, which was 50° from the center of the screen for M1 and 50° or 55° for M2. Trials from the Stim-In and the Stim-Out paradigms were randomly interleaved with each other and with the behavioral trials.
Intracortical electrical stimulations were made in three hemispheres (right for M1, both for M2). The FEF was defined in accordance with physiological criteria established previously (Bruce et al. 1985) and was first mapped in a previously published study (Monteon et al. 2010). Briefly, with the animal's head restrained, we explored the cortex adjacent to the arcuate sulcus. We verified that these sites were within the FEF by electrically evoking saccades from these sites, or immediately adjacent sites, at low current thresholds (Bruce et al. 1985) (300 Hz, 200 ms, <50 μA). After finding a low-threshold site, we released the animal's head and delivered higher currents aiming to recruit eye and head movements (Chen 2006; Monteon et al. 2010; Tu and Keating 2000) (300 Hz, 200 ms, 60–90 μA). Electrode probe locations were confirmed during a postmortem histological examination after the experiments were concluded (Monteon et al. 2010).
All of the stimulation sites described here produced naturally coordinated movements of the eyes and head, as described previously (Monteon et al. 2010). In addition, we excluded sites that produced very large gaze shifts (to match the range in which targets were presented in the behavioral task), and we only analyzed movements from days in which the control behavioral data showed a significant cue-dependent effect, as described in Experiment 1: Cue-Dependent Behavior.
Kinematic Analysis for Behavioral and Stimulation Experiments
Each animal's head was at the center of three mutually orthogonal magnetic fields, voltages from the coil were sampled at 1,000 Hz, and data were digitized and stored on disk. We used these data to compute horizontal and vertical position signals of gaze (eye relative to space), head relative to space, and eye relative to head. Head velocity was calculated based on the third-order 25-Hz low-pass Butterworth-filtered signal. The chosen bandwidth was based on an FFT analysis showing that the frequency components of the head signals from M1, M2, and M3 were all below 10 Hz. Data files for both experiments (behavior and stimulation) were processed in a graphic user interface.
Only successful trials were included in analysis. Trials were rejected if gaze movement anticipated appearance of the target, if the animal performed Out-Out movements in response to the Out-In stimuli (or vice versa), if the animal used more than the minimum number of gaze shifts to acquire targets, or if either the gaze or the head did not move toward the correct target. Such errors were common during early training but remained occasional after training.
Within successful trials, initial and final gaze and head positions were defined by three time epochs: 1) the beginning of gaze or head movements, whichever was the first; 2) the end of the gaze movement; and 3) the end of the head movement; corresponding to the end of the vestibuloocular reflex (VOR) eye movement that follows the gaze shift. Head and gaze movement amplitudes were calculated as the difference between these initial and final positions.
Because of the idiosyncrasies of head movement strategies between animals, directions, conditions, and trials, we could not find a fixed set quantitative detection criterion for final head position that worked for all conditions. Instead, an observer visually selected final head position for the first gaze shift, using the following criteria: 1) If there was no apparent head movement (sometimes in Out-In, rarely in Out-Out), head position at the end of the first gaze shift was selected. 2) If the head moved but then stopped or reversed direction before the second gaze shift (often in Out-In, sometimes in Out-Out), this inflection point was selected. 3) If the head continued moving in the same direction after the first gaze shift but showed a dip in velocity before the second gaze shift (never in Out-In, often in Out-Out), the low point of this velocity dip was selected. 4) If the head continued moving in the same direction between gaze shifts and showed no velocity dip (sometimes in Out-In and often in Out-Out), head position at the onset of the second gaze shift was selected as final head position.
These criteria provide the possibility for a bias in the time of head movement offset between the two paradigms, either because of observer bias or because larger head movements last longer (Freedman and Sparks 1997b). To ensure that this did not bias our results, we checked whether there were significant differences between head movement duration between Out-In and Out-Out tasks and then accounted for any differences (see results). Furthermore, we analyzed peak head velocity before the selected end points as an additional objective kinematic measure.
For a single gaze shift, sometimes the amplitude of head motion up until the end of the gaze shift (head contribution to gaze) is the important variable (Freedman and Sparks 1997b), but for planning the next saccade (Oommen et al. 2004) it is the final head position associated with the preceding gaze shift that is important. Therefore, we primarily used the latter measure but sometimes also analyzed head contribution to gaze for comparison. Note that, in either case, we only analyzed the kinematics of the first gaze shift in this study (corresponding to the epoch between vertical lines 1–3 in Fig. 1, C and D, well before the visuomotor reaction to T2).
Gain increase index and direction calculation.
The gain increase (GI) index was calculated based on the following formula for the behavioral trials: GIb = (HsOut − HsIn)/HsIn, where GIb = GI index for behavioral trials, HsOut = head amplitude for each trial in the Out-Out paradigm, and HsIn = head amplitude for each trial in the Out-In paradigm. The mean GI was calculated by adding the values from individual trials on each target direction and dividing the resultant over the number of observations. For microstimulation, GIs = (HsStimOut − HsStimIn)/HsStimIn, where GIs = GI index for microstimulation trials, HsStimOut = head amplitude for each trial in the Stim-Out paradigm, and HsStimIn = head amplitude for each trial in the Stim-In paradigm. The mean GIs was calculated by adding the values from individual trials on each FEF stimulation site and dividing the resultant over the number of observations.
To pair the trials for the mathematical operations shown above we used a head initial position criterion: for a given experimental session we calculated the initial position for the head movement at the beginning of each trial for the Out-In and Out-Out paradigms. Then we paired each Out-In trial with a corresponding Out-Out trial that has the most similar initial head position to the Out-In trial, and the difference between the two initial head positions must be smaller than 2°. A similar procedure was used for each stimulation site, except that the pairing was done between Stim-In and Stim-Out trials. All data analysis and image processing were implemented with custom-written batch scripts in MATLAB 6.5 (MathWorks). Statistical comparisons included two-tailed Student's t-tests, n-way analysis of variance (ANOVA) tests, Tukey post hoc tests, and ANCOVA tests.
Unit Recording Paradigm and Analysis
After an initial training period of 12 wk on animal M3, using the same techniques described above, we commenced recording in the SC. SC sites were identified through a combination of standard stimulation and recording techniques (Klier et al. 2003; Marino et al. 2008) (the animal is still in use, so histology is not yet available). Unit or multiunit activity was recorded with the use of tungsten microelectrodes and a Plexon system. When gaze shift-related activity was identified in the SC, the motor responses of the cell were rapidly tested in various directions within contralateral space with targets placed at 20° or 50° eccentricity, in order to identify cells that were active for gaze shifts to 20° targets and determine their optimal tuning direction.
The behavioral paradigm (Fig. 1) was then modified so that the direction of T1 was matched to the best tuning vector of the cell. However, T1 was left fixed at 20° in order to obtain a “population response” corresponding to the data in our behavioral experiment; we did not alter it to match the cell's best amplitude tuning. Single-unit/multiunit activity was then recorded with Out-In, Out-Out trials; 6/7 of these were in the on-direction and off-direction, whereas 1/7 of these trials were made in one of the other 6 directions as the distracters, all randomly intermingled.
A total of 101 SC sites were recorded, but the subsequent off-line kinematic analysis revealed that animals did not reach full behavioral proficiency in the task until the 51st recording session. During the first 50 recording sessions, a high proportion of trials did not meet the behavioral inclusion criteria described above, and animals did not yet consistently show the head movement strategy described in results. Therefore, we did a general analysis of all the data (see Fig. 10 for a summary of the 2 main responses observed) but only did a complete quantitative analysis on data collected during recording sessions 51–101 (see Figs. 11 and 12), where the animal showed the effect being studied here. Only the behavioral data associated with these recording sessions were used as the behavioral data for M3, as described above.
Single-unit activity was discriminated off-line through Offline Sorter (Plexon) and Neuromining (lab-developed software). Spike density functions were calculated by using a moving average with a 15-ms window. Unit analysis windows were selected for each neuron to include the event-related burst profile as observed in both paradigms.
To check whether cells had different responses to Out-In and Out-Out conditions in the time period around the first gaze shift, we first calculated the average firing rates during the analysis window in both the Out-In and Out-Out conditions and then applied a Student's t-test over the firing rates of Out-In versus Out-Out with the “left” tail. A similar test was also done in the time period (±100 ms) around the peak of the head displacement for some cells (see results).
To verify whether these activity differences are due to the condition difference, rather than the initial head position bias that possibly happened in the behavior, for each cell we paired the trials of Out-In and Out-Out in the same way we used for GI calculation and applied a paired Student's t-test over the paired trials from all gaze burst cells, with “left” tail.
During our analysis, we found that the distributions of ratios between unit responses in the Out-In and Out-Out paradigms from different recording sessions were not Gaussian. For this reason, the nonparametric Spearman ranked correlation coefficient was used here to estimate correlations between activity and behavior across recording sessions.
When not stated otherwise, P values reported in results were obtained with the two-tailed Student's t-test. Values reported as P ≈ 0 signify values below MATLAB's limit.
RESULTS
Experiment 1: Cue-Dependent Behavior
Three rhesus monkeys, denoted here as M1, M2, and M3, were trained to perform the behavioral paradigms illustrated in Fig. 1, A and B. Note that the animals' head movements during gaze shifts were never constrained in any way—either physically or through task rewards. Animals were simply trained to shift gaze direction using whatever coordination strategy they chose. Importantly, there was no difference between the locations or appearance of T1 in the Out-In and Out-Out paradigms. The required gaze shift was identical; only the cue color and the future events were different. The behavioral data shown here were obtained by randomly interleaving trials from the Out-In and Out-Out paradigms (in all 8 directions), so that monkeys could only predict the final target position from T1 if they learned to associate this with the cue. Note also that we only analyzed the kinematics of the first gaze shift (corresponding to the epoch between vertical lines 1–3 in Fig. 1, C and D, well before the behavioral reaction to T2).
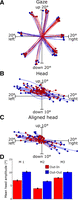
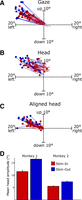
Figure 2 summarizes the main observations and results of this experiment; a more detailed directional analysis follows. Trajectories correspond to all of the trials presented in a single experimental session with trials randomly interleaved between the Out-In (n = 53) and Out-Out (n = 57) trials. Gaze trajectories are roughly similar in regard to the amplitudes and directions in both paradigms. Figure 2B shows the entire head movements associated with these gaze shifts, which tend to be smaller than gaze trajectories, especially along the vertical dimension. The key point is that the head trajectories (Fig. 2, B and C) had smaller amplitudes for the Out-In trials compared with the Out-Out trials (5.07 ± 0.59° and 10.29 ± 0.81°, respectively). This can be seen more clearly in Fig. 2C, where the start points of the same head movements have been realigned to the center of the coordinate axis (see also the example plotted in Fig. 1, C and D).
Basic results of behavioral paradigms. A–C: 2-dimensional gaze and head position trajectories from 1 experimental session, plotted in space coordinates. Filled circles, end points of each movement. Out-In trials (red trajectories) and Out-Out trials (blue trajectories) were randomly intermingled. Gaze trajectories (A) were plotted up until the end of the gaze shift portion of the movement, whereas head trajectories (B) were plotted until the head movement stopped. C: same head position data as B, but the initial positions have been realigned to the origin (0, 0). D: mean head amplitude, averaged across all experimental sessions for the Out-In paradigm and the Out-Out paradigm in the 1st monkey (M1, left), the 2nd monkey (M2, center), and the 3rd monkey (M3, right). Error bars indicate SE.
Figure 2D shows the mean head movement amplitudes (for the entire head movement) for all the population of data collected; 1,027 trials were recorded in 9 sessions for M1, 984 trials were collected in 15 sessions for M2, and 4,657 trials were collected in 36 sessions for M3. For M1 the mean (±SD) total head amplitudes were 7.04 ± 0.26° for the Out-In (n = 496) and 10.20 ± 0.27° for the Out-Out (n = 518) trials. For M2 the mean amplitudes were 3.93 ± 0.11° and 6.56 ± 0.17° for the Out-In (n = 468) and Out-Out (n = 511) trials, respectively. For M3 the mean amplitudes were 7.96 ± 0.14° and 10.09 ± 0.12° for the Out-In (n = 2,252) and Out-Out (n = 2,405) trials, respectively. These differences were highly significant (P ≈ 0) in all three monkeys. These differences remained significant when analyzed separately for each of the 60 experimental sessions (P < 0.05).
Similarly, there was a significant increase in peak head velocity during the head movement associated with the first gaze shift between Out-In and Out-Out in M1 (53.6 ± 1.4°/s vs. 68.9 ± 1.4°/s; P = 5.96e-14), M2 (37 ± 0.66°/s vs. 49.2 ± 1°/s; P ≈ 0), and M3 (50.1 ± 0.72°/s vs. 66.5 ± 0.77°/s; P ≈ 0). This suggests that the observed increase in head amplitude cannot be completely accounted for as an inclusion of a portion of the second rise in a bimodal response.
In comparison, changes in eye-in-head saccade amplitude between Out-In and Out-Out were very small and not significant in the first two animals: M1 (16.2 ± 0.13° vs. 16.1 ± 0.14°; P = 0.457), M2 (17.3 ± 0.23° vs. 17.7 ± 0.24°; P = 0.278), M3 (17.6 ± 0.06° vs. 18.7 ± 0.07°; P ≈ 0). These differences were slightly higher for total gaze amplitude and were significant in two of the three animals: M1 (17.1 ± 0.10° vs. 17.5 ± 0.11°; P = 0.01), M2 (18.5 ± 0.21° vs. 19.0 ± 0.22°; P = 0.135), M3 (15.9 ± 0.07° vs. 16.9 ± 0.06°; P ≈ 0). This slight increase might indicate a greater contribution of head movement during the gaze shift (this is explored more fully below).
To be certain that a change in head movement strategy has occurred, it is necessary to divide head displacement by gaze displacement. When the trial-by-trial ratio of head amplitude over gaze amplitude was taken, there was a significant increase in this ratio from the Out-In to the Out-Out paradigm in all three animals: M1 (0.42 ± 0.016 vs. 0.61 ± 0.017; P = 1.01e-14), M2 (0.23 ± 0.008 vs. 0.38 ± 0.011; P ≈ 0), and M3 (0.48 ± 0.008 vs. 0.58 ± 0.007; P ≈ 0). This shows that the context-dependent head movement increases were not just due to larger gaze shifts; training produced a context-dependent change in eye-head coordination strategy.
It does not appear that these changes in head movement strategy were related to overall differences in initial head position distributions. There were no significant differences between the distributions of initial horizontal head position for all experiments in our three monkeys (M1 P = 0.80, M2 P = 0.63, and M3 P = 0.57). There was no significant difference in vertical head position in two of the three animals (M1 P = 0.77, M2 P = 0.61), although there was a difference in M3 (P = 0.002).
We also tested whether the duration of the full head movement (as defined in materials and methods) accompanying the first gaze shift of the Out-Out paradigm was longer than its counterpart in the Out-In paradigm, and whether this accounted for our results. There was no significant difference in the duration of Out-In versus Out-Out in M3 (250 ± 1.9 ms vs. 249 ± 1.5 ms; P = 0.761). However, there was a significant difference in M1 (224 ± 2.5 ms vs. 278 ± 2.5 ms; P ≈ 0) and M2 (206 ± 2.6 ms vs. 285 ± 3.2 ms; P ≈ 0). This in itself is not inconsistent with our hypothesis, because larger head movements last longer (Freedman and Sparks 1997b), but to ensure that duration alone did not account for the changes in amplitude in M1 and M2, we redid our calculations at the midpoint of the two durations. To do this, we calculated DT, the difference between the average time duration of Out-In versus Out-Out, for each target direction and recalculated head movement amplitudes, using the former end points plus DT/2 ms for Out-In trials and minus DT/2 ms for Out-Out trials (i.e., at the midpoint of the original times for each target direction). This still yielded highly significant differences in head amplitude for Out-In versus Out-Out in M1 (7.4 ± 0.26° vs. 8.96 ± 0.25°; P = 1.53e-5) and M2 (3.98 ± 0.12° vs. 5.45 ± 0.14°; P = 5.88e-15). Thus these differences cannot be explained away by marking bias or different movement durations.
To test whether these effects were direction dependent, we used our GI index for each target direction (see materials and methods). An index greater than zero indicates that the Out-Out paradigm produced larger head movements. To ensure that the cue-dependent effect was not due to differences in initial head position, we matched the data sets according to initial head position.
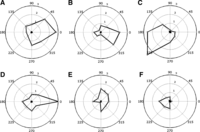
Figure 3 shows the average GI indexes, plotted as a function of target direction in polar coordinates, for both the complete head movements (Fig. 3, A–C) and the head movements up until the end of the gaze shift (Fig. 3, D–F). Note that any nonzero value on the graph indicates an increase of head movement in the Out-Out paradigm relative to the Out-In paradigm. For reference, we also show the similar GI data for gaze amplitude, which cluster around zero, confirming that the Out-Out task does not appreciably increase gaze amplitude in any direction. In contrast, a task-dependent increase in head movement was present for all animals, and the magnitude was not the same for different directions. It appears that the effect was stronger to the right in animals M1 and M2 and stronger to the left in M3 (but note that M3 was simultaneously undergoing neurophysiological testing, primarily in the left visual field).
Direction dependence of gain increase (GI) index in the behavioral paradigms. GI index represents head movement in the Out-Out paradigm relative to the Out-In paradigm. A–F: data points (gray circles) represent average GI index from all trials for each T1 gaze target, plotted as a function of T1 direction in polar coordinates. Top: GI index data computed from the complete head movements for M1 (A), M2 (B), and M3 (C). Bottom: GI index computed from the head movements at the end of the gaze shift for M1 (D), M2 (E), and M3 (F). For reference, GI values for gaze amplitude (black circles) are also provided in each panel; these cluster around zero, showing little or no effect for gaze in any direction. In contrast, all head values were positive (i.e., Out-Out produced larger head movements), except M3 at direction 315°. Tukey post hoc analysis showed that for the complete head movements in M1 (A) the mean GI index (2.75 ± 0.84) at direction 0° was different from the mean GI indexes at directions 180° and 225° (0.61 ± 0.17, 0.63 ± 0.20, respectively). In M2 (B), the mean GI index directions 0° (2.14 ± 0.47) and 315° (2.01 ± 0.31) were different from the mean GI indexes at directions 45°, 90°, 135°, 180°, 225°, and 270° (0.83 ± 0.15, 0.76 ± 0.22, 0.29 ± 0.09, 0.80 ± 0.16, 0.59 ± 0.15, 0.26 ± 0.10, respectively). In M3 (C), the mean GI index directions 180° (2.54 ± 0.04) and 225° (3.65 ± 0.12) were different from the mean GI indexes at directions 0°, 45°, and 90° (0.56 ± 0.01, 0.17 ± 0.01, 0.24 ± 0.01, respectively). In addition, direction 225° was also different from directions 270° and 315° (1.19 ± 0.03, 0.72 ± 0.05). In the case of head movements at the end of the gaze, for M1 (D) the mean GI index directions at 0° (2.14 ± 0.47) and 315° (2.01 ± 0.31) were different from the mean GI indexes at directions 45°, 90°, 135°, 180°, 225°, and 270° (0.83 ± 0.15, 0.76 ± 0.22, 0.29 ± 0.09, 0.80 ± 0.16, 0.59 ± 0.15, 0.26 ± 0.10, respectively). For M3 (F) the mean GI index direction 180° (1.48 ± 0.03) was different from directions 0°, 45°, 90°, and 315° (0.20 ± 0.01, 0.06 ± 0.02, 0.49 ± 0.02, −0.03 ± 0.02, respectively). M2 (E) had no main effect in the ANOVA test; see text for further explanation.
This direction dependence was analyzed by an n-way ANOVA for each monkey (with target direction as a factor: 8 levels, 1 per target direction). For the complete head movements we found that there was a significant main effect for direction in the mean GI index of M1 (Fig. 3A; F = 2.69; P = 9.9 × 10−3), M2 (Fig. 3B; F = 9.32; P = 1.63 × 10−10), and M3 (Fig. 3C; F = 7.41; P = 2.02 × 10−8), i.e., in each animal one or more directions were significantly different from each other. A Tukey post hoc analysis was then used in each case to show which directions were significantly different from each other (see Fig. 3 for details). The same type of analysis was carried out for the head movements at the end of gaze (head contribution to gaze; Fig. 3, bottom). We found a significant main direction effect for the mean GI index in M1 (F = 3.38; P = 1.7 × 10−3) and M3 (F = 5.31; P = 7.59 × 10−6). Post hoc analyses were again used to show which GI indexes were significantly biased by target direction (see Fig. 3 for details). In M2 for the head movements at the end of gaze (Fig. 3E), we found no significant main direction effect difference in mean GI index (F = 1.88; P = 7.3 × 10−2). In conclusion, although the training was symmetrical, the results were direction specific for two of three animals at gaze end and for all three animals at the end of head motion.
Finally, when peak head velocities associated with the first gaze shift were analyzed separately for each target direction, every direction showed a significant increase from the Out-In to the Out-Out condition in M1 (P ≤ 1.7 × 10−3), M2 (P ≤ 4.5 × 10−4), and M3 (P ≤ 3.1 × 10−5). Thus all directions were affected in some fashion, but to different extents in the position domain.
Experiment 2: Cue Dependence During FEF Stimulation
The next question we asked was whether the FEF is involved in triggering these context-dependent behaviors, specifically, whether this requires a context-dependent modulation within FEF (unlikely to be evoked by electrical stimulation) or whether a fixed FEF output can trigger such behavior. In a previous study we found that stimulation of these same FEF areas in the same animals (before training) evoked normally coordinated eye-head gaze shifts (Monteon et al. 2010). Here we examined the effects of training on the Out-In and Out-Out cues on these eye-head coordination patterns.
After animals M1 and M2 had been trained on the preceding behavioral paradigms and we had established that they had learned to associate the cue with different head movement strategies, we explored the FEF with microstimulation. A total of 53 stimulation sites (M1: 32, M2: 21) were studied in the left and right FEF of two head-unrestrained monkeys. We first verified each FEF saccadic site by delivering low-threshold (300 Hz, 60 pulses, 200 ms, <50 μA) stimulation currents that produced stimulation-evoked saccades (Bruce et al. 1985) with the monkeys head fixed. Of the 53 FEF sites tested, 26 met the inclusion criterion described in materials and methods (14 M1 and 12 M2). With the Stim-In condition as the baseline condition (because it is most similar to standard head-unrestrained stimulation protocols), these 26 stimulation sites produced gaze shifts with latency range of 48.9 ± 7 ms to 189 ± 22 ms (M1) and 20.4 ± 1.4 ms to 51.6 ± 3 ms (M2), with amplitude ranges of 9.77 ± 1.6° to 17.4 ± 3.7° (M1) and 11.6 ± 0.9° to 41.4 ± 0.4° (M2). The remainder of this section provides explicit comparisons between the Stim-In and Stim-Out conditions for these and several other key parameters, especially head motion.
During the FEF experiment, monkeys continued to perform the randomly interleaved Out-Out paradigm and Out-In paradigm as described above. However, randomly, on one of every three trials, the following occurred: instead of showing T1, the fixation light (cue) was followed by a gap period and then stimulation of the FEF. The sequence of events for the stimulation paradigms and two typical stimulation trials (Stim-In and Stim-Out) are shown in Fig. 4, A–D. In this case, only the stimulation-evoked movements were analyzed. The key logical point here is that in the two stimulation paradigms the site and the parameters of stimulation (300 Hz, 60 pulses, 200 ms, set current) were exactly identical and no target was presented; the only difference was the color of the (now extinguished) cue.
Stimulation paradigms. Same conventions as Fig. 1. A and B: sequence of experimental events. These paradigms were interleaved randomly in 1/3 of the trials with the behavioral paradigm. Colored symbols indicate the LED targets at each interval; the room was otherwise dark. At the beginning of each trial monkeys fixated at a central color cue for 300 (M1) or 450 (M2) ms. A: Stim-In paradigm; the central cue was red. When the cue was extinguished, the monkey was required to continue fixating (GAP) for 210 (M1) or 340 (M2) ms before a 200-ms train of electrical pulses was delivered to the right or left frontal eye field (FEF). B: Stim-Out paradigm; the central cue was green (shown here as blue). When the cue was extinguished, the monkey was required to continue fixating (GAP) for 210 (M1) or 340 (M2) ms before a 200-ms train of electrical pulses was delivered to the right or left FEF. C and D: horizontal position trajectories of gaze and head are plotted as a function of time. These trajectories belong to monkey M1 and were recorded during stimulation of the same FEF site. C: Stim-In paradigm. D: Stim-Out paradigm. Colored areas depict the time in which the color cue was shown. Numbered vertical lines represent 3 time epochs used to analyze the data: 1, the beginning of gaze or head movements (whichever was first); 2, the end of the gaze movement; and 3, the end of the head movement. G, gap; S, stimulation; D, delay period.
Figure 5 summarizes the main observations and results from this experiment using the same conventions as Fig. 2; more detailed analysis follows in Figs. 6⇓–8. Figure 5, A–C, show typical data from the Stim-In and Stim-Out paradigms. In this case, gaze shifts (Fig. 5A) only go in one direction (the direction specified by 1 particular FEF site: left-up). The head movements (Fig. 5, B and C) are noticeably larger for Stim-Out (6.52 ± 0.82° and 10.50 ± 1.02°, respectively). This can also be observed in the example shown in Fig. 4, C and D.
Basic results of stimulation paradigms. Same conventions as Fig. 2. A–C: 2-dimensional gaze and head position trajectories from 1 FEF site, plotted in space coordinates. Filled circles, end points of each movement. Stim-In trials (red trajectories) and Stim-Out (blue trajectories) were randomly intermingled with behavioral controls. Gaze trajectories (A) were plotted up until the end of the gaze shift portion of the movement, whereas head trajectories (B) were plotted until the head movement stopped. C: same data as in B except that the initial positions were set to the origin of the coordinate system (0, 0). D: mean head amplitude, averaged across all experimental sessions for the Stim-In paradigm and the Stim-Out paradigm in the 1st monkey (M1, left) and the 2nd monkey (M2, right). Error bars indicate SE.
Figure 5D shows the mean amplitudes of the full head movement for all of the evoked trajectories, averaged across sites during Stim-In and Stim-Out trials. For M1 the mean amplitudes were 11.40 ± 0.41° and 16.90 ± 0.46° for the Stim-In (n = 227) and Stim-Out (n = 272) trials, respectively. For M2 the mean amplitudes were 5.29 ± 0.15° and 7.34 ± 0.27° for the Stim-In (n = 204) and Stim-Out (n = 250) trials, respectively. These differences were highly significant in both monkeys (M1 P ≈ 0 and M2 P = 6.10e-10). Analyzing on a site-by-site basis, we found that all of our 26 stimulation sites produced a greater mean head amplitude in the Stim-Out condition compared with the Stim-In condition. This increase was statistically significant (P < 0.05) in 21 (11 M1, 10 M2) of these sites. Finally, there was a significant overall increase in peak head velocity between Out-In and Out-Out in both M1 (84.3 ± 2.8°/s vs. 104 ± 2.9°/s; P = 5.5 × 10−7) and M2 (56.5 ± 1.3°/s vs. 66 ± 1.8°/s; P = 2.5 × 10−5).
Our experimental design makes no clear prediction about the cue dependence of gaze and eye amplitude in the stimulation condition. There was no physical target in our stimulation paradigm, so the evoked gaze shift was entirely open-loop. Gaze amplitude could be altered by either underlying changes in head amplitude or eye amplitude, which in turn could be influenced by general excitability or anticipation of larger head movements (Crawford and Guitton 1997), but if eye/gaze amplitudes are altered, one needs further tests to show a change in coordination.
We found that task-dependent changes in eye and gaze amplitude were proportionately smaller than the changes in head amplitude described above but still significant. Comparing Stim-In and Stim-Out for eye-in-head, the values were 8.29 ± 0.36° vs. 11.0 ± 0.39° (P = 4.49e-7) for M1 and 23.9 ± 0.74° vs. 25.8 ± 0.62° (P = 0.043) for M2. For gaze, the values were 13.4 ± 0.43° vs. 18.4 ± 0.50° (P = 4.21e-13) for M1 and 27.0 ± 0.75° vs. 29.7 ± 0.65° (P = 0.008) for M2.
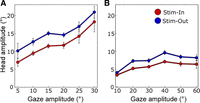
We therefore required a direct test to address the possibility that changes in head amplitude were only a by-product of changes in gaze amplitude (Freedman and Sparks 1997b). In contrast to our behavioral data, the stimulation data were composed of a range of different gaze amplitudes within and across sites. Here, the accepted standard is to plot head amplitude as a function of gaze amplitude (Fig. 6). As in our previous papers (Constantin et al. 2004; Martinez-Trujillo et al. 2003) we generated these plots by pooling the data from each condition and then grouping it in bins (5° for M1 and 10° for M2) sorted by gaze amplitude to obtain means and standard deviations. Figure 6 compares the plots for Stim-In and Stim-Out in both M1 (Fig. 6A) and M2 (Fig. 6B). For both animals and for every bin, the mean head movement amplitude was greater in the Stim-Out paradigm compared with the Out-In paradigm. To test the difference between the groups in Fig. 6, we used an ANCOVA test to fit a regression line to data in each group (Stim-In and Stim-Out). The test revealed that the regression line for the Stim-Out group is significantly elevated over the regression line of the Stim-In group (F = 29.56, P = 8.54 × 10−8 and F = 42.10, P = 2.01 × 10−10 for M1 and M2, respectively). These data show that the cue-dependent effect was proportionately greater for the head at all gaze amplitudes.
Head movement amplitude as a function of gaze shift amplitude for evoked gaze shifts in the FEF Stim-In trials and Stim-Out trials for M1 (A) and M2 (B). Data from all stimulation sites were pooled and put into 5° (M1) or 10° (M2) bins according the size of gaze shift. Paradigm effect was found highly significant (ANCOVA, P < 0.01; see text for details). Error bars indicate SE.
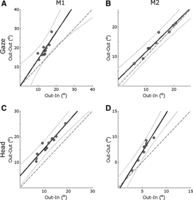
To determine how the influence of the Stim-Out cue on gaze kinematics depends on baseline movement amplitude in the Stim-In condition, we did an across-site regression of eye, head, and gaze amplitude for Stim-Out as a function of Stim-In. As shown in Fig. 7 for gaze (Fig. 7, top) and head (Fig. 7, bottom), the relationships were highly linear. Linear correlation coefficients were 0.948, 0.956, and 0.819 (M1) and 0,979, 0.959, and 0.977 (M2) for eye, head, and gaze respectively. There was a trend toward an increased slope for gaze in M1 (Fig. 7A) but not M2 (Fig. 7B). The confidence intervals for the gaze regression either mostly (M1; Fig. 7A) or completely (M2; Fig. 7B) contained the slope = 1.0 line within the data range. In contrast, the confidence intervals for the head regression either completely (M1; Fig. 7C) or mostly (M2; Fig. 7D) excluded the line of equality within the data range. Note that this was for two different reasons: in M1 (Fig. 7C) there was a shift in the head intercept, whereas in M2 (Fig. 7D) there was an increase in head slope. This might reflect different neural strategies in M1 versus M2 (either strategy works for the target amplitude used in training).
Across-site regression of Stim-Out vs. Stim-In mean evoked gaze and head amplitudes. A and C: animal M1. B and D: animal M2. Top: gaze amplitude. Bottom: head amplitude. Gray circles, mean amplitudes for each site. Black and gray solid lines, regression fits ± 95% confidence intervals; dashed line, slope = 1 and intercept = 0.
It does not appear that our cue-dependent stimulation results were caused by differences in overall distribution in initial head position. No significant differences were found in either the horizontal plane (2-tailed Student's t-test, M1 P = 0.97 and M2 P = 0.84) or the vertical plane (M1 P = 0.86 and M2 P = 0.87). The same was true when the 21 stimulation sites were analyzed on an individual basis (P < 0.05).
We also quantified the duration of the head movement that accompanied the first gaze shift and again checked to make sure that this did not bias our results. We found that the duration of head movements that accompanied the first gaze shift was significantly different between the Stim-In and Stim-Out paradigms in both M1 (225 ± 4.7 ms vs. 299 ± 3.8 ms; P ≈ 0) and M2 (177 ± 3.7 ms vs. 215 ± 2.4 ms; P ≈ 0). We therefore retested the head amplitudes for the two paradigms at their temporal midpoint, in the same way described above for the behavioral data. We found that head amplitude was still significantly larger in the Stim-Out versus the Stim-In paradigm in both M1 (15.4 ± 0.44° vs. 11.8 ± 0.41°; P = 5.96e-9) and M2 (7.09 ± 0.27° vs. 5.52 ± 0.15°; P = 2.53e-6). Thus this effect was not simply due to the longer duration of head movement in Stim-Out.
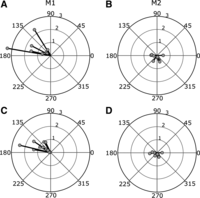
To quantify whether cue-dependent head movement effects were position-, direction-, or site-dependent, we matched the data trials according to initial head positions and then we calculated a mean GI index for each FEF site (same conventions as in the analysis of behavioral trials). Figure 8 shows the average GI index for each stimulation site as a vector, plotted in polar coordinates as a function of the average evoked gaze direction for each FEF site. The same scale is used as in Fig. 3. Although the overall context-dependent effect of FEF stimulation was significant at the end of head movement (Fig. 8, top) and at the end of the gaze shift (Fig. 8, bottom) in both monkeys, the GI indexes for M1 were generally higher than for M2.
Site dependence of GI index in FEF stimulation paradigms. Conventions and scales similar to behavioral data in Fig. 3. A–D: each line represents a vector scaled by the GI index of a FEF site, plotted as a function of the average direction of the evoked gaze shift in polar coordinates. Top: GI index vectors when computed from the complete head movements for M1 (A) and M2 (B). Bottom: GI index vectors when computed from the head movements at the end of the gaze shift for M1 (C) and M2 (D). The GI indexes for all FEF sites were positive, i.e., the Stim-Out paradigm always produced larger head movements than Stim-In. Tukey post hoc analysis showed that for the complete head movements in M1 (A) the mean GI index at site 1 (3.36 ± 0.94) was significantly different from the mean GI indexes at sites 3, 4, 5, 6, 7, 10, and 11 (0.26 ± 0.11, 0.31 ± 0.12, 0.41 ± 0.13, 0.31 ± 0.13, 0.31 ± 0.09, 0.50 ± 0.22, 0.64 ± 0.33, respectively). For the head movements at the end of the gaze in M1 (C), the mean GI index at site 7 (0.03 ± 0.13) was significantly different from the mean GI index at site 9 (2.43 ± 1.04). For M2 (B and D) neither the complete head movements nor the movements at the end of gaze end of the gaze had a statistically significant main effect in the ANOVA test; see text for further explanation.
The sensitivity of the GI index to individual sites was analyzed by an n-way ANOVA for each monkey (with stimulation site as a factor: 11 levels for M1 and 10 for M2, 1 level per site in each monkey), and a Tukey post hoc test was applied when a main effect was found. For the complete head movements, we found that for M1 (Fig. 8A) there was a significant main effect for the mean GI index (F = 4.23; P = 3.15 × 10−5). Post hoc analyses showed that the GI index was significantly biased by site (see Fig. 8 for details). Similarly, for the head movements at the end of gaze (head contribution to gaze), in M1 (Fig. 8C) we found a significant main effect difference in mean GI index (F = 1.98; P = 3.8 × 10−2). Post hoc analyses showed that the GI index was significantly biased by site (see Fig. 8 for details). In the case of M2, we found no significant main effect for site on the mean GI index, either for the complete head movement (Fig. 8B) or for the head movement at the end of gaze (Fig. 8D) (F = 1.77, P = 7.6 × 10−2 and F = 1.12, P = 3.5 × 10−1, respectively). To summarize, the effect was significantly site (or direction or day) dependent in M1 but not M2.
In the case of our stimulation data, peak head velocities associated with the first gaze shift were significantly altered in every direction where we obtained data (for this analysis gaze data were grouped according to nearness to 45° direction increments in polar coordinates). Specifically, M1 showed a significant increase from the Out-In to the Out-Out condition for the up, up-left, and left target directions (P ≤ 3.1 × 10−5) and M2 showed a significant increase for right, left, down, and down-right targets (P ≤ 3.0 × 10−3). Other directions could not be tested from this data set.
Finally, gaze movements evoked in the Stim-Out condition showed a trend toward reduction in latency compared with the Stim-In paradigm in both animals. This trend reached significance in M1 (77.3 ± 3.3 ms vs. 67.4 ± 2.9 ms; P = 0.023) but not M2 (35.2 ± 1.0 ms vs. 32.6 ± 1.7 ms; P = 0.210). This might reflect greater intrinsic excitability after the Stim-Out cue (see next section).
Experiment 3: Cue-Dependent Superior Colliculus Activity
On the basis of our behavioral experiment, we expected that the brain must show cue-dependent, predictive activity in this task, but this experiment could not predict the nature or distribution of these signals. Our stimulation experiment adds one constraint, by pointing toward the subcortical targets of the FEF. To demonstrate the existence of such physiological signals, and to gain insight into the nature of these signals, we recorded from the SC in our third animal (M3), using a modified version of our behavioral task (Fig. 1). Recall that the behavioral data from these recordings sessions were used in the behavioral analysis provided for M3 above, so this has already been documented and will not be described again.
Overview of SC unit responses.
As described in materials and methods, we recorded activity from 101 SC sites, for the purpose of identifying modulations in gaze-related burst activity. Seventeen of these sites were rejected from analysis because they showed unstable or highly noisy responses or behavioral errors/parameter changes were made during the experiment. Twenty sites showed fixation-related activity (with a pause in activity during saccades) and were not further analyzed.
From the remaining 64 sessions, we were able to discriminate 81 separate signals. Seventeen of these signals were rejected because they showed inseparable multiunit activity and/or heterogeneous responses that were difficult to classify. One unit was rejected from analysis because it showed a response during the ipsilateral return movement (thus all remaining cells were only active for movements in the contralateral “on-direction”). The remaining 63 units showed two types of motor burst responses, as described below (Figs. 9 and 10). (Twelve of these units also showed a visual response to the appearance of the target, but this was not analyzed here.)
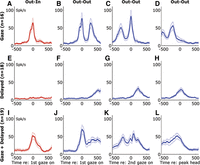
Forty-two of the motor burst cells showed activity just before and during the first gaze shift in the sequence. A typical example of a cell that showed a gaze-related burst is shown in Fig. 9A. This cell showed a robust burst of action potentials during the first gaze shift in both behavioral paradigms (Fig. 9, A and B). We called this the “gaze burst” response. Twenty of these 42 cells only showed a gaze burst and no other response, like classic gaze-burst cells (Marino et al. 2008). None of these cells showed a visual burst. Note that T1 was not matched to the receptive field peak for any cells, so the maximum firing rate for all gaze-related activity was likely underestimated. We therefore used a criterion modified from Marino et al. (2008) for quantitative inclusion of these responses for further analysis. We only included cells that reached at least 50 spike/s above baseline, within a 3-ms window between −20 ms and 0 ms before first gaze saccade onset. This quantitative criterion reduced this data set to 16 cells. Visual inspection confirmed that all of these 16 cells showed a typical burstlike profile that peaked just before and/or after the first gaze onset (as in Fig. 9A). We called these “gaze-burst” neurons.
Representative examples of a neuron that showed a gaze-related burst (top) and another neuron that only showed a delayed burst (bottom), plotted during trials from the Out-In condition (left, red) and the Out-Out condition (right, blue). A: example of a neuron that showed a gaze burst in the Out-In condition. Top: the trial-aligned raster and the average spike density function, plotted as a function of time. FR, firing rate. Middle and bottom: corresponding gaze and head positions. Red and black trajectories represent the horizontal and vertical components, respectively, of the gaze shift or head movement. x-Axis is time aligned relative to the 1st gaze shift onset in ms. B: the same neuron recorded in the Out-Out condition. Here, the horizontal components are represented in blue (to denote the cue). Note that this neuron also showed an additional prolonged “delayed burst” in the Out-Out condition, but it is not clear what event this relates to. C: example of a neuron that only showed a delayed burst (not visible here in the Out-In condition). x-Axis is time aligned relative to the peak of the head movement. D: the same neuron in the Out-Out condition, where the delayed burst does appear and is not temporally related to any gaze shift.
The remaining 22 cells that showed a gaze burst response also showed prolonged activity after the second gaze shift in the Out-Out paradigm (Fig. 9B). We treated these as a separate population, described below (Fig. 10, bottom); 19 of these fulfilled our criteria for analysis.
Summary of 53 neurons that showed a gaze-related burst (A–D, n = 16), a delayed burst (E–H, n = 18), or both responses (I–L; n = 19). Mean spike density functions (±95% confidence intervals) are aligned to the onset of the 1st gaze shift (1st column) for the Out-In condition (red traces) and aligned to the onset of the 1st gaze shift (2nd column), the onset of the 2nd gaze shift (3rd column), and peak head displacement (4th column) for data collected in the Out-Out condition (blue traces).
There was also an unexpected group of 21 cells that did not show activity related to any amplitude or direction of gaze shift, unless they were accompanied by a large contralateral head movement (for example, the 50° return movement following ipsilateral Out-Out trials—not shown here). Moreover, these cells responded for excursions of head position in their on-direction in the absence of a gaze shift. For example, within our main task paradigms they showed a delayed burst during the second fixation period of the Out-Out task (Fig. 9D). Note that this activity peaked well after (>150 ms) the second gaze shift in the cell's on-direction, and well before (>150 ms) the return movement. This is unlikely to be preparatory activity for the final return movement, because 1) the activity fades instead of growing as this movement approaches, 2) SC cells rarely prepare for ipsilateral movements, and 3) these cells indeed were not active during ipsilateral gaze/head movements (not shown). In these cells, the temporal profile of the delayed response bore a remarkable resemblance to the positional excursion of the head after the second outward saccade. However, since we did not systematically vary head vs. gaze responses, we called this a “delayed-burst” response. Three of these 21 cells looked too “noisy” for analysis, so 18 were retained for further quantification.
Figure 10 documents the gaze-burst and delayed-burst responses from the 53 motor burst neurons that fulfilled all of the criteria provided above and shows how they appeared together in some neurons. This figure shows mean spike density functions (±95% confidence intervals) using several different temporal alignments for the following three cell populations.
Figure 10, top, shows the 16 neurons that only showed a gaze burst response. These show the classic gaze-related burst in the Out-In condition (Fig. 10A) and behaved exactly as expected in the Out-Out condition: they showed two clearly separated bursts for gaze shifts in their “on-direction,” the first related to the first gaze shift (Fig. 10B) and the second burst related to the second gaze shift (Fig. 10C). Note that these two bursts are offset by ∼400 ms and the second starts ∼200 ms after the first gaze shift. This activity was complete when the head reached its peak excursion (Fig. 10D), and again they did not show any other responses.
Figure 10, middle, shows the 18 neurons that showed only the delayed response (as illustrated in Fig. 9, C and D). Again, these were inactive in the Out-In condition (Fig. 10E) but in the Out-Out condition showed a burst of activity after the first (Fig. 10F) and second (Fig. 10G) gaze shifts, instead peaking at the same time as the peak excursion of head position in the “on-direction” (Fig. 10H). This burst was in decline by the onset of the return saccade in the “off-direction” (not shown).
Figure 10, bottom, shows the third class of 19 neurons like the example shown in Fig. 9B. Plotted in this way, it is now more clear that these neurons showed characteristics of both types of response described above, i.e., activity in Fig. 10, bottom, is essentially the sum of activity shown in Figure 10, top and middle. There are minor exceptions to this rule: the gaze-related burst in the Out-In condition (Fig. 10I) shows a slightly prolonged “tail” that is not present in Fig. 10A or Fig. 10E, and this tail might be more prominent in the Out-Out condition (Fig. 10J). The second gaze burst also appears to be lower (Fig. 10K). However, it is difficult to discriminate signals in these cells after the first of the Out-Out gaze shifts, because the second gaze burst is likely mixed with the delayed response.
Overall, this preliminary analysis suggested that there were only two major responses in our motor burst neurons, a gaze burst and a delayed (perhaps head related) burst that could each appear in isolation (Fig. 10, top and middle) or in combination (Fig. 10, bottom). We were unable to detect any difference in the anatomic distributions of these response types.
Quantification of unit responses vs. cue-dependent head movement strategy.
Here we return to the specific purpose of this experiment: our paradigm was designed to test cue-dependent activity capable of predicting head movements related to the first saccade to T1. We only expected to see the gaze-burst activity observed in the standard Out-In condition (Fig. 10, A, E, and G) and aimed to correlate this with cue-dependent modulations in the first head movement. As described in materials and methods, only experiments corresponding to our final 51 SC recording sessions showed a significant (P < 0.05) cue-dependent modulation of the head movement strategy (see experiment 1 above for details of this modulation). Seventeen of our 35 gaze-burst cells were recorded within this period. Only two of these cells showed a pure gaze burst response, whereas the majority showed the combined gaze burst and delayed burst responses, so these neurons were combined and we did not analyze the gaze burst separately for these populations. The experiment was not designed to test activity during the second saccade, and gaze/delayed burst activity was not separable at that time (see Fig. 10K), so we only analyzed the gaze burst associated with the first gaze shift in the Out-In versus Out-Out paradigms.
When designing our experiment we did not expect to see the “delayed burst” described above, but this response was too prevalent to be ignored so we used this as a “control group” for comparison with the gaze burst response. Again, in neurons that showed the combined response it was not possible to separate the gaze burst related to the second saccade from delayed (head) related activity in the Out-Out condition (Fig. 10, K and L), so we only analyzed the delayed response in “pure” delayed-burst cells. Of our 18 “pure delayed” cells, 11 fell within the period in which there was a significant cue-dependent behavioral modulation.
We now focus on task-related differences (Out-Out vs. Out-In) in these two response types. Note that in the examples shown so far, the gaze-burst cell appeared to be more active (for the first outward gaze shift) in the Out-Out paradigm (Fig. 9A) compared with the Out-In paradigm (Fig. 9B) and the delayed-burst cell was almost silent in the Out-In paradigm, only becoming active in the Out-Out paradigm. We now quantify these modulations in the 17 gaze-burst cells and 11 delayed-burst cells that were recorded during significant behavioral modulation.
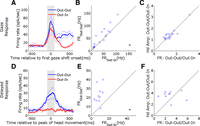
Figure 11 documents these observations for our final population of gaze burst responses (Fig. 11, top) and for our final population of delayed burst responses (Fig. 11, bottom). Figure 11, left, shows mean (±95% confidence intervals) interspike frequencies across all cells, aligned on the start of the first gaze saccade for gaze-burst cells (Fig. 11A) and aligned at the time of the peak excursion of head movement for delayed-burst cells (Fig. 11D). Comparing Out-Out to Out-In, and matching trials according to initial head position as in our behavioral experiment, there was a significant increase in action potential frequency during the selected window for both gaze-burst cells (P = 0.0023) and delayed-burst cells (P = 1.53 × 10−11). Figure 11, center, plots the mean frequency for individual cells in the Out-Out condition as a function of their mean frequency in the Out-In condition (over the same time window for each neuron). Both gaze-burst cells (P = 0.0023) and delayed-burst cells (P = 0.0048) showed a population distribution of firing rates skewed toward the Out-Out paradigm.
Population responses in Out-Out vs. Out-In conditions. Only data that met both our unit and behavioral training criteria are shown. A: mean (±95% confidence intervals) spike density functions for all neurons that showed the gaze burst response. Gray zone shows the average time period of the windows in which the firing rates in B and C were calculated. B: scatterplot of the firing rates of each gaze-burst neuron in Out-Out condition as a function of Out-In condition. C: scatterplot of the ratio indexes of gaze-burst neurons. x-Axis is the ratio of the firing rate in Out-Out over the firing rate in Out-In (derived from B). y-Axis is the ratio of the 1st head movement amplitude in Out-Out over that in Out-In. D: mean (±95% confidence intervals) spike density functions for neurons that only showed the delayed-burst response. Gray zone shows the window in which the firing rates in E and F were calculated. This window is defined as ±100 ms of the peak head movement. E: scatterplot of the firing rate of the delayed-burst neurons. F: scatterplot of the ratio indexes of the delayed-burst neurons. Definitions of the ratios are same as those in C.
Thus both cell types were modulated by the task, but did either do so in a way that could predict the behavior? Figure 11, right, correlates neural activity with daily fluctuations in behavior. The ratio of head displacement Out-Out/Out-In associated with the first gaze shift for gaze-burst cells (Fig. 11B) or peak head movement for head-burst cells (Fig. 11E) is plotted as a function of the ratio of the simultaneously recorded action potentials (from the previous column), for each cell. Comparing these two ratios, the gaze-burst cells were good linear predictors of the context-dependent behavior (r = 0.72; P = 0.0012, Spearman correlation coefficient), whereas the delayed-burst cells (r = 0.097; P = 0.777) were poor linear predictors.
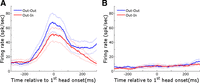
Finally, we checked whether the context-dependent activity of either response type came early enough to anticipate the context-dependent divergence in head movement strategies associated with the first gaze shift. Figure 12A plots population activity as in Fig. 11A, except aligned on first head movement onset. These cells showed cue-dependent divergence in activity >150 ms before head movement. In contrast, the delayed-burst cells (Fig. 12B) only diverged well after the first head movement onset. Thus, while both types were modulated by the task (including a robust and consistent delayed burst response), only gaze-burst neurons showed both the timing and behavioral correlations required to play an anticipatory role.
Gaze burst and delayed burst activity aligned with time of the 1st head movement onset. A: mean spike density functions (±95% confidence intervals) of Out-Out and Out-In conditions for neurons that showed the gaze burst response. B: mean spike density functions (±95% confidence intervals) for neurons that only showed the delayed burst.
DISCUSSION
The preceding results show that 1) monkeys optimize eye-head coordination patterns to suit predicted gaze behavior, 2) they can associate a visual cue with such patterns, 3) these rules are preserved during fixed activation of most FEF sites, 4) both gaze-burst and delayed-burst SC cells are modulated in this paradigm, but 5) only gaze-burst cells showed cue-dependent responses that came before (and correlated with) the first predictive head movement.
Behavioral Implications
Our behavioral experiment showed that, like humans, monkeys can adjust their head movement strategy based on expectation of future gaze direction. When monkeys “expect” to make a future gaze shift in the same direction, the current gaze shift is accompanied by a faster, larger head movement. A similar motor phenomenon was previously demonstrated in humans with the use of repetitive blocks of similar gaze shift sequences (Oommen et al. 2004). However, here we show for the first time that monkeys can make such adjustments from one moment to the next, based on a contextual cue that is completely independent from previous or current motor goals, in this case a learned association between a color cue and future gaze targets.
Most studies of eye-head coordination have emphasized lawful relationships between stimuli and kinematics (Freedman and Sparks 1997b; Guitton et al. 1990; Tomlinson and Bahra 1986), often describing the parameters that determine the “gain” of head movement as a function of gaze shift amplitude (Freedman 2001; Guitton et al. 1990; Lefèvre et al. 1992). The present study illustrates that this parameter is not fixed, but rather is adjustable to support the requirements of expected sequences of gaze position (Oommen et al. 2004). Note that we did not directly control this parameter, i.e., we only provided predictive information and then allowed monkeys to choose their own head movement strategy, presumably for the expected future target. Apparently it was easier (more energetically efficient) for the head to move less when a return gaze shift was expected, whereas a larger head movement was optimal for a succession of same-direction gaze shifts. A larger head movement produces a larger VOR eye movement after each saccade, so that the eye is centered in the orbit and ready for another saccade in the same direction. This would prevent a situation in which a relatively slow head movement is required to acquire the next target. It is likely that humans use similar mechanisms to switch between behaviors such as reading (Proudlock et al. 2003), object manipulation (Castiello 2005), or driving (Land 1992). It is also likely that the motion of the body (McCluskey and Cullen 2007) contributes to such anticipation to orient in large workspaces, as in many sports.
The direction-dependent effects that we observed could not be implemented by adjustment to a single gain factor (Freedman 2001; Guitton et al. 1990; Lefèvre and Galiana 1992). It appears that head amplitude was based on both the direction of the first saccade and the expected final gaze position. This requires head gain and bias terms for both horizontal and vertical movement directions (Tweed 1997). Our first two monkeys showed a bias toward greater rightward head movements in the Out-Out condition, whereas the third animal showed a bias to the left. Possible contributing factors were that 1) our monkeys have a tendency to look toward a lab door to the right between trials, 2) M3 was often recorded while mapping receptive fields on the left, and 3) the juice reward was generally to the left. However, these potential factors do not invalidate any of our main conclusions; they just show that these anticipatory effects are complex.
Previous psychophysical studies have demonstrated visually triggered predictive oculomotor behaviors in humans (Antes and Penland 1981; Nodine and Kundel 1987) and monkeys (Bichot et al. 1996). Other studies have shown that head contribution to gaze can be adjusted to meet physical constraints (Ceylan et al. 2000; Coimbra et al. 2000; Monteon et al. 2005; Stahl 2001) and during repetitive sequences of gaze shifts in the human (Oommen et al. 2004). Our study is the first to combine these approaches, i.e., to show that moment-to-moment changes in visual cues can trigger different eye-head coordination strategies. This provides an experimental model for real-world situations (like those described above), where the influence of visual cues likely depends on both experience and intent. By doing this with monkeys, we were then able to investigate potential neural mechanisms for this complex phenomenon, as discussed below.
Interpretation of FEF Microstimulation Experiment
The second contribution of this study is the observation that cue-dependent eye-head coordination strategies are preserved in FEF stimulation-evoked gaze shifts. One cannot know the exact patterns of neural activation produced by our pulse trains (Ranck 1975). For example, fibers of passage may be affected, there may be antidromic effects, and adjacent areas may be affected (although we find that the site specificity of the behavioral effects is very precise; Monteon et al. 2010). However, this did not matter for our experimental design. What matters is that, regardless of the details, we were inputting a fixed train of electrical impulses to the brain at a fixed location, with only one difference: the color of the cue. We found that in trained monkeys the presence of this cue was sufficient to create an internal state of activity/connectivity sufficiently robust to be accessed by even a crude input with a fixed temporal profile.
The FEF has previously been implicated in other context-dependent oculomotor behaviors. For example, FEF units can show color selectivity after training (Bichot et al. 1996) and stimulation-evoked movements were influenced by a subsequent direction judgment (Gold and Shadlen 2000). The FEF is also involved in coding pro- versus antisaccades (Everling and Munoz 2000) and saccade sequences (Phillips and Segraves 2010). Here we show that FEF stimulation can produce gaze shifts with context-dependent eye-head coordination strategies.
The slight reduction in movement latency that we observed after the Stim-Out (vs. Stim-In) cue suggests that these cues modulated general excitability in the gaze control system, but this alone does not account for the relative changes in head motion relative to gaze amplitude. This cue-dependent effect could occur either through 1) interactions with intrinsic FEF activity or 2) interactions of a fixed FEF output with signals elsewhere in the brain. The first option would require a capacity to multiplex eye and head signals within the FEF and evoke this during microstimulation. It is possible that our stimulation trains recruited adjacent sites that preferentially evoke head movement (Chen 2006), but we thoroughly explored the FEF in both of our monkeys and did not find such responses (Monteon et al. 2010). Another possibility is that head contribution is encoded in FEF action potential patterns (as we observed in the SC), but it is unlikely that our fixed suprathreshold stimulation trains could reproduce such patterns.
Electrical microstimulation activates axons both orthodromically and antidromically, but the former effects are likely more robust (Watson et al. 1982; Yeomans 1990). Furthermore, FEF stimulation might activate earlier gaze control centers through their reciprocal connections, but direct stimulation of earlier gaze centers like LIP requires much higher thresholds to evoke movement and does not appear to evoke head motion (Constantin et al. 2009; Thier and Andersen 1998). It is likely that the main influence of FEF activation on gaze behavior occurs through its direct connections to the SC and areas of the reticular formation involved in eye and head control (King et al. 1980; Komatsu and Suzuki 1985). Although it is possible that some of these signals differentially control eye and head movement (Chen 2006; Constantin et al. 2004), most studies suggest that frontal cortex is primarily involved with encoding desired gaze position (Knight and Fuchs 2007; Martinez-Trujillo et al. 2003; Monteon et al. 2010), leaving the details of eye-head coordination for subcortical structures (Sparks et al. 2001, 2002).
Thus we believe the simplest interpretation of our stimulation data is that the effect was produced by a fixed output, mainly from the FEF, to the brain stem. This does not preclude the presence of more complex context-dependent patterns of FEF activity in normal behavior, but it shows that this is not necessary in our task. This suggests that after training our animals were able to set up a context-dependent “coordination state” at some point downstream from the FEF.
Interestingly, when Stim-Out vs. Stim-In context-dependent head amplitudes were compared across stimulation sites (Fig. 7D), the two animals showed two different strategies that were not evident (or would be equal) in our behavioral data. One animal showed a bias in increased head movement amplitude, whereas the other showed an increased gain of head movement amplitude. Both strategies would work equally in the behavioral task we used to train and test the animals, because the task only used one target amplitude. However, this raises the possibility that head gain and bias could be manipulated separately to suit more complex tasks.
Possible Role of the SC
Our SC recording experiment in animal M3 was originally intended to supplement our stimulation experiment by providing an “existence proof” for cue-dependent signals downstream from the FEF that could be used to modulate the head strategy. The intent was to look for cue-dependent modulations in the gaze burst, which we found, as well as some unexpected results.
First, the gaze burst response in the SC showed cue-dependent signals related to predictive head movement strategies. These “gaze-burst” cells likely correspond to the saccade-related visuomotor and motor cells described in many SC studies (Gandhi and Katnani 2011; Goldberg and Wurtz 1972; Mays and Sparks 1980; Munoz and Guitton 1991; Wurtz and Albano 1980). Surprisingly, although the first gaze shift was identical in our Out-In and Out-Out paradigms, these “gaze-burst” units showed task-related modulations that anticipated and correlated with the task-related head motion. With both our unit recording and behavioral criteria in place, we were only left with 17 cells in this category, so we could not analyze whether this signal is unique to certain burst neuron types. Nor can we be certain that the increased burst is driving the head movement; this could be a cross-correlation with some other factor. However, this finding provides an “existence proof” demonstrating that “gaze burst” responses can carry additional task information that could potentially be used to modulate predictive head motion.
The more unexpected finding was that the majority of our motor burst cells (43/63) showed a robust delayed burst related to large excursions in head position in the Out-Out task. These “delayed burst” responses could not account for anticipatory behavior (the explicit goal of our experiment), but they were clearly task related, showing a huge modulation between our two paradigms. Interestingly, these responses would likely never be observed in a standard head-free gaze shift task like our Out-In condition. Indeed, approximately half of these cells (15/31) were completely silent during the Out-In task. These “pure delay” cells would be missed altogether in standard centrifugal gaze shift tasks. Moreover, similar delayed responses appeared in more than half of our “gaze-burst” cells. In standard gaze shift tasks these cells would simply be called “gaze-burst” cells, and their dual signal nature would remain hidden. One possible clue to identifying these cells in more standard tasks is the observation that they had a longer tail of activity during the gaze burst (Fig. 10I), but we do not know whether this tail would persist in head-restrained recordings. Thus it does not appear that we have discovered a new cell type, but rather a new response that does not show up in standard gaze control paradigms.
The huge modulation between the delayed response in our two behavioral paradigms, and its temporal coincidence with the peak of head amplitude, suggest a relationship between this response and head movement. However, our paradigm was not designed for trial-by-trial correlations between neural activity and behavior, so we cannot make this claim. Our “pure” delayed-burst cells might correspond to a class of neurons in the SC whose activity correlates better to head movement than gaze movement (Walton et al. 2007). The latter do not appear to directly control head motion during gaze shifts (Walton et al. 2008). Similar responses have also been recorded in the mesenchephalic reticular formation (Pathmanathan et al. 2006a, 2006b), which connects with SC (Chen and Wise 1995; Cohen and Buttner-Ennever 1984). Finally, we cannot establish whether our delayed burst response controls head movement or simply reflects sensory feedback about head position. It might also be source independent, like head direction cells in the rodent thalamus (Shinder and Taube 2011). Such an SC signal could be useful for a variety of functions, including internal knowledge of heading direction, postural adjustments for changes in head position, and position-dependent gaze kinematics (Freedman and Sparks 1997a), e.g., for the centrifugal gaze sequences used here. Since we only recorded from one animal and did not design our experiment to test this response, a more thorough investigation is required to understand the delayed “head” response.
We previously found that a fixed electrical stimulus input to the SC does not preserve learned context-dependent eye-head coordination patterns in another task (Constantin et al. 2004). Since the latter adaptation task involved a mechanical occlusion of the visual range rather than a self-selected, predictive strategy, the present task might involve different neural mechanisms, but assuming for the moment that these two tasks do involve similar mechanisms, together they suggest that a natural profile of SC activity and/or parallel cortical input is required. For example, the modulations that we observed in the SC gaze burst could explain the changes that we saw in head velocity (Munoz et al. 1991), and prolonged SC stimulation (analogous to our prolonged delayed responses) produces longer, larger head movements (Freedman et al. 1996).
Neuromuscular Mechanisms for Anticipatory Head Movement Strategies
A central remaining question is, overall, what motor mechanism actually provides for larger (and faster) head movements for the same gaze target, based on anticipation of future gaze plans? One possibility tested here is that a new cortical plan is required for eye and head movements in each trial. As discussed above, our FEF stimulation experiment suggests that a different cortical plan is not required for each movement, at least once the visual cue is processed.
At the opposite extreme, the mechanism could involve cue-dependent “priming” of background levels of neck muscle activation (Corneil et al. 2007, 2008). Direction-specific muscle priming (Rezvani and Corneil 2008) could easily occur in the Oommen et al. (2004) experiment, which used repetitive sequences of gaze shifts in blocks. Muscle priming would be more complex in our experiment, because head rotation cues were provided visually in random order, and only afterwards was the direction of the target specified. The temporal gap between the cue and target presentation was long enough (≥400 ms) to produce a general (directionally nonspecific) priming (Chapman and Corneil 2011), but it is currently unknown how such a general priming would influence the next movement. The visual target itself could influence muscle EMG in a cue-dependent fashion within 75–100 ms (Corneil et al. 2008), but this burst is thought to have relatively little influence on the head movement compared with later motor EMG activity (Chapman and Corneil 2011). Moreover, our finding that the SC gaze burst correlated with cue-dependent modulations in the first movement shows that an additional neural signal is available at that time. Overall, it seems unlikely that priming can entirely explain our data, but to test this hypothesis directly it would be necessary to record neck muscle EMG in our task and compare cue-dependent changes in EMG before and during the actual head movement.
The complement to priming is release from motion inhibition, i.e., release from the neck muscle suppression and cocontraction signals used to stabilize head position (Goonetilleke et al. 2010). It is quite possible that large head movements are the “default” state of the system and that head movement is suppressed in our Out-In paradigm, reading, and in many other social/behavioral contexts (Proudlock et al. 2003). Most of the comments made above about the context dependence and timing of priming also apply to disinhibition. Neither increased nor decreased neck muscle activation alone can explain such behaviors, because they must be compensated by opposite eye movement adjustments to achieve the same gaze shift (the VOR is sometimes suppressed during gaze shifts; Cullen et al. 2004; Guitton 1992; Lefèvre et al. 1992). Thus some gate is required that apportions between eye and head drives (the main focus of this and most other eye-head coordination studies) and, if this mechanism involves head movement suppression, some high-level signal for that suppression. For example, high-threshold stimulation of parietal cortex produces saccades but appears to suppress head movement (Constantin et al. 2009). It is possible that the cue-dependent SC modulations that we observed are involved in relaying the signal that disengages suppression of head motion.
Another possibility to consider is that cue-dependent head movement depends on the partial superimposition of two successive neural drives from the gaze control system. This hypothesis can be evaluated with both our behavioral and unit recording data. In our Out-Out behavioral data, sometimes the head came completely to rest by the time of the second gaze shift, but in many cases there was no clear line between the first and second head movements. Thus the beginning of the second movement could have influenced the kinematics of the first movement. However, the effect was already present at the end of the first gaze shift, and we also observed a significant modulation of peak head velocity associated with the first gaze shift, so it is unlikely that only the end of the head movement was affected. Moreover, this hypothesis would require a second gaze command to arrive in time to influence the head kinematics of the first movement. In our SC data, the second gaze burst developed after the head early modulations described above (Fig. 10B) and the delayed response developed much later (Fig. 12). Our combined gaze-delayed burst neurons (Fig. 10J) showed an elongated burst that may have modulated the late movement, but this does not appear to be a separate gaze command. The cue-dependent modulation that we saw in our SC gaze burst data suggests a different mechanism.
Taken together, our data suggest that the cue-dependent behavior derives from cue-induced patterns of excitability and gating (which we called “coordination states” above) within the brain stem gaze control system. This hypothesis suggests that once these states are set up by some cue-dependent mechanism, a fixed cortical command (as in our FEF stimulation data) would trigger different patterns of brain stem activity (as in our SC unit recording data). This hypothesis does not conflict with a role for neuromuscular mechanisms such as priming and suppression (because neck muscle activity is, of course, set by the brain stem and spinal cord), in conjunction with additional commands during the movement. To investigate this hypothesis, it would be useful to record downstream from the SC within the premotor circuitry for eye-head control (Crawford et al. 2003; Pathmanathan et al. 2006a, 2006b; Sparks et al. 2001).
What Triggers These Cue-Dependent States?
Another intriguing topic is the mechanism that allows a visual cue to trigger changes in motor coordination strategy. Most visuomotor studies investigate the relationship between the goal and the movement; less is known about the neural pathways that might allow for contextual influence on motor coordination strategy. A task like ours that involves rapid adjustments of eye-head gating based on a random color cue would require a network spanning both cortex and brain stem, perhaps analogous to the networks for cue-dependent task switching in pro- versus antisaccades (Chapman and Corneil 2011; Everling and DeSouza 2005). In particular, the prefrontal cortex (PFC) receives complex visual inputs (Perecman and Institute for Research in Behavioral Neuroscience 1987), codes signals related to current task demands (Duncan 2001; Miller and Cohen 2001), and has reciprocal connections with both gaze structures (Huerta et al. 1987) and the cerebellum, which is implicated in motor learning (Ramnani 2006). PFC is thought to influence processing in other parts of the brain in accordance with current task demands (Miller and Cohen 2001). For example, PFC shows rule-dependent oculomotor responses (Everling and DeSouza 2005) that are relayed directly to the SC (Johnston and Everling 2006). Thus a structure like the PFC may process context-dependent cues and use this information to influence eye-head gating in lower-level motor control centers.
GRANTS
This work was supported by a Canadian institutes of Health Research grant to J. D. Crawford. J. A. Monteon was supported by CONACYT. M. Avillac was supported by the Human Frontier Science Program. J. D. Crawford was supported by the Canada Research Chair Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A.M., M.A., X.Y., H.W., and J.D.C. conception and design of research; J.A.M., M.A., X.Y., and H.W. performed experiments; J.A.M. and X.Y. analyzed data; J.A.M., X.Y., and J.D.C. interpreted results of experiments; J.A.M. and X.Y. prepared figures; J.A.M., X.Y., and J.D.C. drafted manuscript; J.A.M., M.A., X.Y., H.W., and J.D.C. edited and revised manuscript; J.A.M., M.A., X.Y., H.W., and J.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Saihong Sun for programming assistance and Dr. Brian Corneil for comments related to neck muscle priming.
- Copyright © 2012 the American Physiological Society