Research / Molecular Evolution
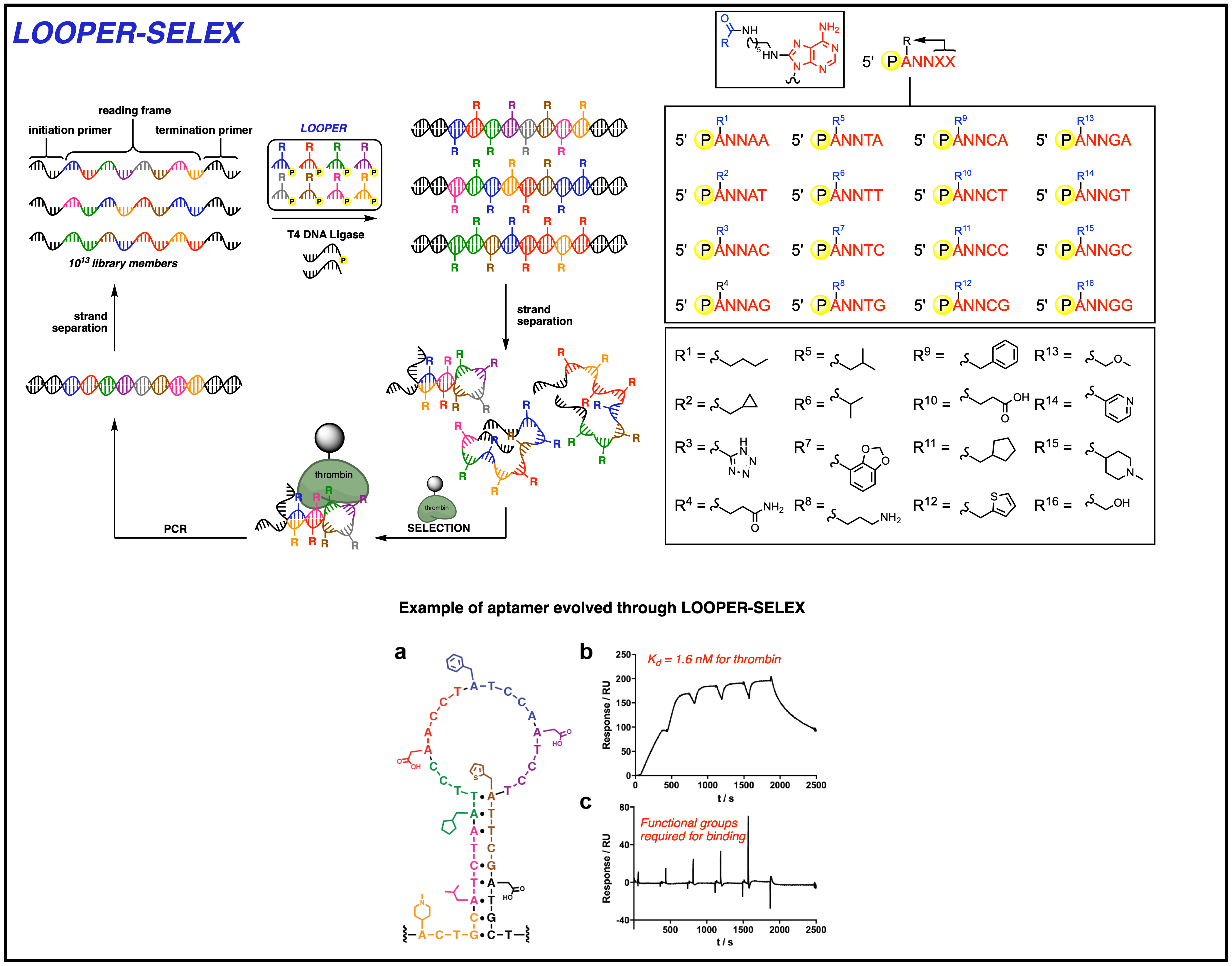
We are interested in developing new methods to evolve DNA aptamers with significantly increased chemical diversity, in order to increase their functional capacity as receptors and catalysts. In the rapidly growing fields of proteomics and glycomics, in-depth analysis of protein expression, modifications, and interactions on a genomic scale has required ready access to high affinity binding ligands as tools for discovery. While antibodies have traditionally served in this important role, their numerous shortcomings, which include slow production time, high cost, limited target availability, poor stability, variable production quality, and unknown specificity profiles, have prompted enthusiasm in developing alternative high-affinity ligands for biomedical research. Nucleic acid aptamers, which obviate the issues presented with antibodies, have recently come to the fore to address these concerns with promising success. Aptamers are single-stranded nucleic acid polymers that have been evolved through in vitro selection methods to bind to desired molecular targets. While successful for a variety of targets, nucleic acids have limited chemical diversity, which many believe has limited the potential of this polymer to serve as a high-affinity ligand. To address this challenge, our lab developed a technology called Ligase-catalyzed OligOnucleotide PolymERization (LOOPER). This method enables the high-fidelity sequence-specific modification of DNA with up to 256 different chemical modifications. Importantly, this method works with large libraries (1013) of DNA sequences. We have shown that LOOPER can be used to evolve tight binding and highly thermostable aptamers against human alpha thrombin, displaying tight binding and inhibition of proteolytic activity. Our lab continues to improve the LOOPER system and explore it capacity to generate highly decorated biopolymers with unique functional properties.