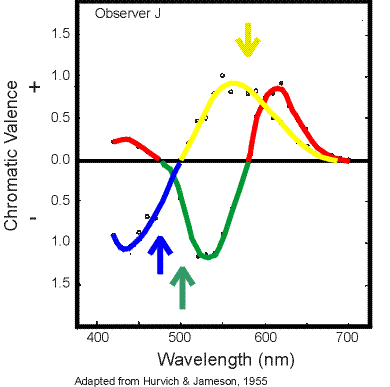
 The figure on the left helps to show how monochromatic lights appear. The arrows point to the wavelengths that, for observer J, appear as unique yellow, green, and blue. Unique red can not be obtained with a single wavelength. Even at 700 nm a small amount of yellow is apparent. To obtain unique red one must take a long wavelength (e.g. 700 nm) and mix it with a little bit of blue to cancel the yellow. Recall that a hue is unique when only one hue is perceived. So a unique occurs where the chromatic valence of the two other hues is zero.
The figure on the left helps to show how monochromatic lights appear. The arrows point to the wavelengths that, for observer J, appear as unique yellow, green, and blue. Unique red can not be obtained with a single wavelength. Even at 700 nm a small amount of yellow is apparent. To obtain unique red one must take a long wavelength (e.g. 700 nm) and mix it with a little bit of blue to cancel the yellow. Recall that a hue is unique when only one hue is perceived. So a unique occurs where the chromatic valence of the two other hues is zero.The positive and negative valences are arbitrarily assigned. They are assigned to represent the opponent characteristics of the colors.
the chromatic valence represents the amount of color seen at different wavelengths. For example, at 450 nm the blue valence is approximately -1.0 and the red valence is approximately 0.2. These valences indicate that the perceived color should be blue with a small red component. This color is usually called violet or purple. At 550 nm there is a large yellow and a large green chromatic valence indicating that this color should appear greenish yellow, which is what most people see.
Table of Contents
Subject Index
Table of Contents [When not using frames]