U n i v e r s i t é Y O R K U n i v e r s i t y
ATKINSON FACULTY OF LIBERAL AND PROFESSIONAL STUDIES
SCHOOL OF ANALYTIC STUDIES & INFORMATION TECHNOLOGY
S C I E N C E A N D T E C H N O L O G Y S T U D I E S
NATS 1800 6.0 SCIENCE AND EVERYDAY PHENOMENA
ATKINSON FACULTY OF LIBERAL AND PROFESSIONAL STUDIES
SCHOOL OF ANALYTIC STUDIES & INFORMATION TECHNOLOGY
S C I E N C E A N D T E C H N O L O G Y S T U D I E S
NATS 1800 6.0 SCIENCE AND EVERYDAY PHENOMENA
Lecture 9: How Old is It?
| Prev | Next | Search | Syllabus | Selected References | Home |
Topics
-

Splendid Isolation on the East Side of Patriarch Grove, White Mountains
One of the most significant turning points in science was the discovery that the age of the Earth (and therefore of the universe) was much greater than religious texts claimed. Probably the most important figure in this respect is Charles Lyell, a British geologist, whose "most important specific work was in the field of stratigraphy [ … ] From 1830 to 1833 his multi-volume Principles of Geology was published. The work's subtitle was 'An Attempt to Explain the Former Changes of the Earth's Surface by Reference to Causes now in Operation,' and this explains Lyell's impact on science. He was, along with the earlier John Playfair, the major advocate of the then-controversial idea of uniformitarianism, that the earth was shaped entirely by slow-moving forces acting over a very long period of time. This was in contrast to catastrophism, a geologic idea that went hand-in-hand with age of the earth as implied by biblical chronology. In various revised editions (twelve in all, through 1872), Principles of Geology was the most influential geological work in the middle of the 19th century, and did much to put geology on a modern footing." [ from Charles Lyell (Wikipedia) ] Lyell proposed several processes, such as sedimentary deposition, erosion and volcanism, by which the various kinds of rocks are transformed over time. Since fossils were very much part of the puzzle, he introduced new ideas and new methods in the fledgling area of paleontology, suggesting for example that certain types of fossils, reference fossils, could be used to mark specific periods of geological time. Clearly such methods, still in use today, are not sufficiently precise to permit an accurate determination of the age of other fossils and of human artifacts. The tools needed had to wait until well into the twentieth century, when our understanding of atomic and nuclear science, and radioactivity in particular, opened up a whole range of precise techniques. -
Radiometric dating is the name of a series of technologies which allow the determination
of the age of certain materials by analyzing the decay of radioactive isotopes that were incorporated into
the material at the time of its formation. These methods assume that such isotopes did not leach out since,
and also that the initial proportions of the radioactive isotopes, as well their decay products, are known.
Such conditions are met by certain radioactive processes such as Argon-Argon, Carbon-Nitrogen, Potassium-Argon,
Rubidium-Strontium, Samarium-Neodymium, Uranium-Thorium, etc. I will illustrate here what is probably, historically,
the first such method. This method, generally known as Carbon-14 Dating was first proposed
by Willard Frank Libby, who in 1960 was awarded the Nobel Prize in Chemistry precisely "for his method
to use carbon-14 for age determination in archaeology, geology, geophysics, and other branches of science."
"Carbon has two stable isotopes: carbon-12 ( 12C ), and carbon-13 ( 13C ). In addition, there are tiny amounts of the unstable isotope carbon-14 ( 14C ) on earth. 14C has a half life of just under 6000 [ 5730 ± 40 ] years, and so would have long ago vanished from the earth, were it not for its constant formation by cosmic ray impacts on nitrogen in the atmosphere. When cosmic rays enter the atmosphere they undergo various transformations, including the production of neutrons. The resulting neutrons participate in the following reaction: n + 14N → 14C + 1H This reaction is relatively common, as nitrogen constitutes nearly 80% of Earth's atmosphere. The highest rate of carbon-14 production takes place at altitudes of 30,000-50,000 feet, and at higher geomagnetic latitudes, but the carbon-14 spreads evenly throughout the atmosphere and reacts with oxygen to form carbon dioxide. Carbon dioxide also permeates the oceans, dissolving in the water. Since it is assumed that the cosmic ray flux is constant over long periods of time, carbon-14 is assumed to be continuously produced at a constant rate and therefore that the proportion of radioactive to nonradioactive carbon throughout the Earth's atmosphere and oceans is constant. Plants take up atmospheric carbon dioxide by photosynthesis, and are eaten by animals, so every living thing is constantly exchanging 14C with its environment as long as it lives. Once it dies, however, this exchange stops, and the amount of 14C gradually decreases through radiocative decays 14C → 14N + e- The maximum range of radiocarbon dating appears to be about 50,000 years, after which the amount of 14C is too low to be distiguished from background radiation." [ from Radiocarbon Dating (Wikipedia) ]
It is important to note that Libby's assumption that the rate of production of Carbon-14 is essentially constant over long periods of time turned out to be incorrect, because the intensity of the cosmic rays which produce it varies significantly due to the varying conditions of the upper atmosphere and the radiation belts of the Earth. The much-publicized depletion of the ozone layer also contributes to the variation of Carbon-14 production. Luckily, other independent techniques allowed the calibration of the Carbon-14 method.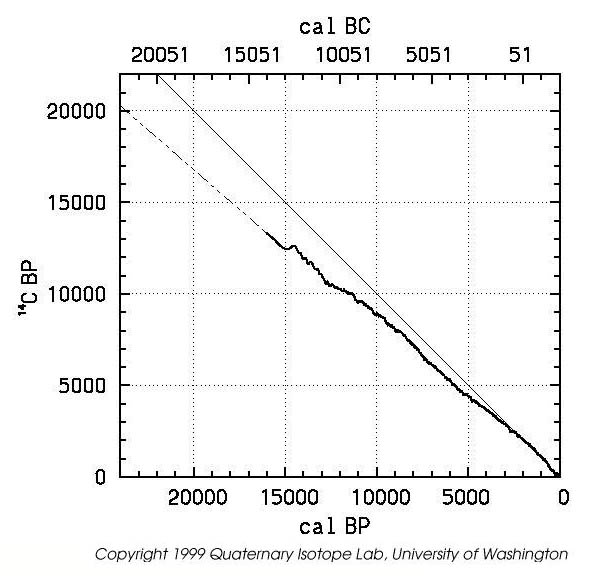
Calibrated Radiocarbon Master Timescale
-
One of the methods used to cross-check and calibrate the accuracy of the Carbon-14 scale was dendrochronology,
"a method of dating wooden objects by analyzing the pattern of their annual rings and comparing this pattern
to an established tree-ring sequence in the region." [ from Dictionary of Science and Technology (AP, 1992) ]

Section of a SW White Pine with Scar from an 1842 Wildfire
"A fantastic close-up picture that shows how tree rings can be used to date when during the growing season the wildfire occurred (photo © H D Grissino-Mayer). This shows a section of a southwestern white pine (Pinus strobiformis) found on Mt. Graham in southern Arizona. The fire occurred in the early portion of the earlywood (EE) of 1842, probably in May or June." [ from Picture Gallery of Trees and Tree Rings ]
In particular, the technique of cross-dating a large number of cores extracted from several long-lived trees such as the bristle-cone pine was crucial in the calibration of the Carbon-14 method.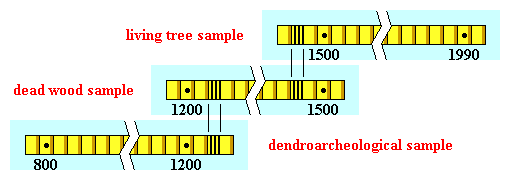
Schematic Representation of the Cross-Dating Method
Since the oldest bristlecone pine specimen found to date, and appropriately nicknamed Methuselah, is 4,767 years old, cross-dating allowed the precise calibration of the Carbon-14 Method in the range from 2750 BC to the present (other methods extended this range well into the past). "The bristlecone pine chronology in the White Mountains currently extends back almost 9,000 years continuously. That's to 7,000 BC! Several pieces of wood have been collected that will extend this date back even further. The hope is to push the date back to at least 8,000 BC. This will be important as the last Ice Age ended about 10,000 years ago, and to have a record of this transition period would offer scientists a wealth of information." [ from Dendrochronology ]
Dendrochronology ]
-
Dendrochronology and Carbon-14 Dating can only be used with wood and other organic materials. How do we date
artifacts, such as pottery, tools, etc., or rocks? As you can see from the references below, there are many
methods, some of which rely on techniques similar to those used with the Carbon-14 method. One promising method is
thermoluminescence dating. "Thermoluminescence (TL)
is the process in which a mineral emits light while it is being heated. The light emitted is due to the recombination
of charges trapped at metastable defect sites within the mineral lattice, and its amount is proportional to energy
absorbed by the mineral as a result of its previous exposure to ionizing radiation."
"Normally, charges trapped in deep traps in a mineral's lattice will remain there indefinitely if the mineral rests at the Earth's ambient surface temperatures. However, heating a mineral to 500°C immediately evicts trapped electrons from the deep traps. They promptly undergo recombination, and in doing so emit photons in the visible spectrum. For any given mineral, the amount of light emitted varies in a reproducible manner with temperature; a graph of this relationship is called the glow curve. The amount of light emitted is proportional to the mineral's intrinsic sensitivity to radiation and the total amount of radiation energy absorbed. Comparisons made of the light emitted by a natural sample and the amount of light emitted by a sample with a known added radiation dose, plus separate dosimetry measurements, allow the sample's age since it was last exposed to a clock-resetting event to be calculated." [ from thermoluminescence ]
This method allows the determination of the age of previously heated materials up to about half a million years ago, and can be applied not only to pottery, ceramics, bricks, etc., but also to volcanic material. -
I will conclude this all-too-brief and selective review of dating methods with a look at the predicatble question:
how old is the universe itself? A good place to begin is NASA's How Old is the Universe?.
In general, if you research this topic, you will find that one of the first answers to your question relies on the
observation that the universe is expanding. If we measure the rate of expansion (closely
related to the famous Hubble constant) by extrapolating back in time we should be able to calculate when the universe
began. This is more complicated than it first appears, because the rate of expansion depends on whether the universe is
essentially empty or not of matter. This is clear if we remember that gravity, a property of matter, is an attractive force,
which would then slow down the expansion, particularly when the universe was more dense than it is now. This method also
assumes somehow that this rate has been constant, something we have many reasons to doubt. The currently accepted
age of the universe determined with this method is between 12 and 14 billion years.
Another method is to study special populations of stars.
"The life cycle of a star depends upon its mass. High mass stars are much brighter than low mass stars, thus they rapidly burn through their supply of hydrogen fuel. A star like the Sun has enough fuel in its core to burn at its current brightness for approximately 9 billion years. A star that is twice as massive as the Sun will burn through its fuel supply in only 800 million years. A 10 solar mass star, a star that is 10 times more massive than the Sun, burns nearly a thousand times brighter and has only a 20 million year fuel supply. Conversely, a star that is half as massive as the Sun burns slowly enough for its fuel to last more than 20 billion years. Astronomers can place a lower limit to the age of the universe by studying globular clusters. Globular clusters are a dense collection of roughly a million stars. Stellar densities near the center of the globular cluster are enormous. If we lived near the center of one, there would be several hundred thousand stars closer to us than Alpha Centauri, the star nearest to the Sun [ … ] All of the stars in a globular cluster formed at roughly the same time, thus they can serve as cosmic clocks. If a globular cluster is more than 10 million years old, then all of its hydrogen burning stars will be less massive than 10 solar masses. This implies that no individual hydrogen burning star will be more than 1000 times brighter than the Sun. If a globular cluster is more than 2 billion years old, then there will be no hydrogen-burning star more massive than 2 solar masses." [ from How Old is the Universe? ]
A very different and more recent method relies again on radioactivity, specifically that of Uranium. "Astronomers have spotted for the first time the fingerprint of uranium-238 in an ancient star—and have used it to make the most reliable guess yet of the age of the universe. Roger Cayrel of the Observatoire de Paris-Meudon, France, and colleagues have used a kind of 'stellar carbon dating' to estimate the age of the star—and therefore the minimum age of the universe. The new estimate makes the universe 12.5 billion years old—give or take 3 billion years." [ from Uranium Reveals the Age of the Universe ] The trick here is to focus on so-called metal-poor stars. These are usually stars which formed early in the history of the universe, when 'the building material' was essentially only hydrogen. The only traces of heavier elements were the result of then rare supernovas. It is therefore relatively easy to measure the amount of radioactive uranium-238 present in such metal-poor stars, and estimate how much of it has already decayed. Are the results of these three types of measurement consistent? The situation is getting better, but until recently there were disturbing discrepancies among them. In a nutshell, the globular cluster method seemed to suggest that these clusters are older than the universe! Something is definitely amiss. "This contradiction implies that either 1) our measurement of the Hubble constant is incorrect, 2) the Big Bang theory is incorrect or 3) that we need a form of matter like a cosmological constant that implies an older age for a given observed expansion rate." [ from How Old is the Universe? ] Fortunately, more recent measurements seem now to converge towards a plausible value of 13.7 ± 0.2 billion years. Very recent experiments from the Luna project in Italy, however, suggest that The Universe, Seen under the Gran Sasso Mountain, Seems to Be Older than Expected : "'As a consequence, in the light of Luna’s new data, the age of our Universe passes from the previous estimate of about 13 billions years to that of about 14 billions years' explains Eugenio Coccia, director of Gran Sasso National Laboratories."
Readings, Resources and Questions
- The Scout NSDL Report for the Physical Sciences has a very useful set of links on Radiometric Dating
- A well-organized, clear, and rather comprehensive site is Dating Techniques at MSU EMuseum, Minnesota State University in Mankato, MN. Dating Methods in Archaeology reviews many of the modern dating methods, and offers concise but useful examples. Chemistry of Art and Artifacts is the website of a course offered at Carlton University. Read especially the Course Notes 1 through 6. If you are interested in pursuing dendrochronology further, consult the rather complete (10,000+ references!) and searchable Bibliography of Dendrochronology.
-
Visit Henri D. Grissino-Mayer's Ultimate Tree-Ring Web Pages
at the University of Tennessee. A marvelous website, "designed to be the ULTIMATE source for information on
the science of Dendrochronology."
Visit The Ancient Bristlecone Pine for
more detailed information about this precious tree and its habitat.
For a nice, non-technical account, read
 How Carbon-14 Dating Works
An interesting article appeared in a recent issue of American Scientist. Read Dating Ancient Mortar
How Carbon-14 Dating Works
An interesting article appeared in a recent issue of American Scientist. Read Dating Ancient Mortar
- A good section on thermoluminescence is included in the Dating Mathods in Archaeology page suggested above. Interestingly, the author notes that "thermoluminescence has also been successfully used to detect forgeries in antique ceramic art collections."
-
Wikipedia offers a very useful
 Timeline of the Universe.
Timeline of the Universe.
- As you are probably aware by now, it is very difficult to determine the age of the universe. Browse through Age of the Universe. This tutorial is rather technical, but gives you a rather precise sense of what it takes to estimate how old the cosmos is. Notice also that several other methods are not mentioned here.
© Copyright Luigi M Bianchi 2003-2005
Picture Credits: Leonard Miller · U niversity of Arizona · UBC · University of Tennessee
Last Modification Date: 02 November 2005
Picture Credits: Leonard Miller · U niversity of Arizona · UBC · University of Tennessee
Last Modification Date: 02 November 2005